institute for health transformation (InHealth)
Joaquim Cardoso MSc
Chief Researcher, Editor and CSO
January 26, 2023
Abstract
What is the problem?
- SARS-CoV-2 infection is clinically heterogeneous, ranging from asymptomatic to deadly.
- A few patients with COVID-19 appear to recover from acute viral infection but nevertheless progress in their disease and eventually die, despite persistent negativity at molecular tests for SARS-CoV-2 RNA.
What was the scope of the study?
- Here, we performed post-mortem analyses in 27 consecutive patients who had apparently recovered from COVID-19 but had progressively worsened in their clinical conditions despite repeated viral negativity in nasopharyngeal swabs or bronchioalveolar lavage for 11–300 consecutive days (average: 105.5 days).
What are the findings?
- Three of these patients remained PCR-negative for over 9 months.
- Post-mortem analysis revealed evidence of diffuse or focal interstitial pneumonia in 23/27 (81%) patients, accompanied by extensive fibrotic substitution in 13 cases (47%).
- Despite apparent virological remission, lung pathology was similar to that observed in acute COVID-19 individuals, including
- micro- and macro-vascular thrombosis (67% of cases),
- vasculitis (24%),
- squamous metaplasia of the respiratory epithelium (30%),
- frequent cytological abnormalities and syncytia (67%), and
- the presence of dysmorphic features in the bronchial cartilage (44%).
- Consistent with molecular test negativity, SARS-CoV-2 antigens were not detected in the respiratory epithelium.
- In contrast, antibodies against both spike and nucleocapsid revealed the frequent (70%) infection of bronchial cartilage chondrocytes and para-bronchial gland epithelial cells.
- In a few patients (19%), we also detected positivity in vascular pericytes and endothelial cells.
- Quantitative RT-PCR amplification in tissue lysates confirmed the presence of viral RNA.
What are the conclusions?
- Together, these findings indicate that SARS-CoV-2 infection can persist significantly longer than suggested by standard PCR-negative tests, with specific infection of specific cell types in the lung.
- Whether these persistently infected cells also play a pathogenic role in long COVID remains to be addressed.
- Together, these findings indicate that SARS-CoV-2 infection can persist significantly longer than suggested by standard PCR-negative tests, with specific infection of specific cell types in the lung.
- Whether these persistently infected cells also play a pathogenic role in long COVID remains to be addressed.
INFOGRAPHIC
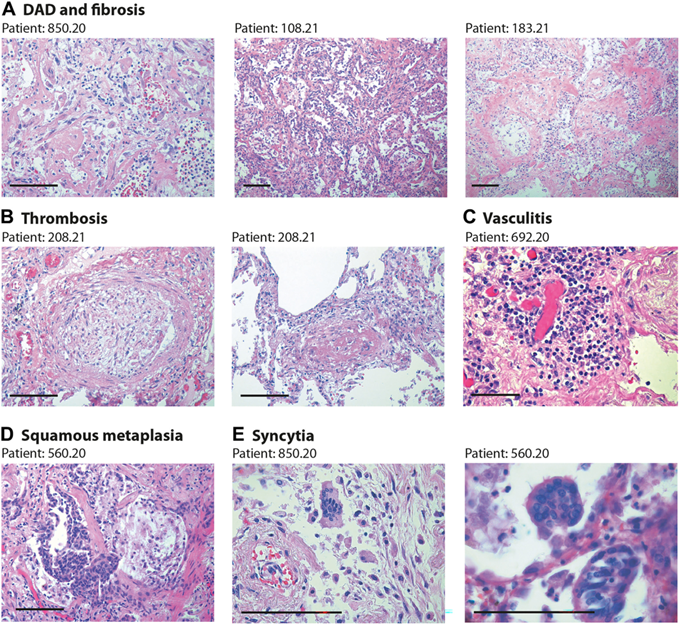
Figure 1
Lung pathology in former COVID-19 patients. Haematoxylin and eosin (H&E) staining showing characteristic pathological features of previous COVID patients (patient’s code is at the top of each panel). Scale bars in all panels: 100 μm. (A) Diffuse alveolar damage (DAD) and parenchymal fibrosis. The pictures, taken from three different patients, show massive alveolar inflammation with almost complete occlusion of the alveolar lumen; delamination of pneumocytes, which appear grossly damaged and dystrophic; endoalveolar fibrin deposition; and fibrosis of the inter-alveolar septa, which is massive in patient 183.21. (B) Thrombosis of a medium- (left) and small- (right) sized artery. The thrombotic artery on the left is surrounded by hyalin fibrotic tissue and flanked by congested small vessels. Thrombi are in different stages of organisation. © Perivascular inflammatory cap in a small thrombotic vessel. (D) Squamous metaplasia (pseudosyncytia) of the respiratory epithelium. Massive defoliation of the metaplasic alveolar cells, only a few of which maintain a cylindrical ciliated appearance. Massive congestion of the surrounding small vessels. (E) Presence of syncytial cells in two patients. In the upper panel, the syncytium is in the context of a delaminated alveolar structure and in proximity to an arteriole with a thickened wall and exfoliated endothelium.
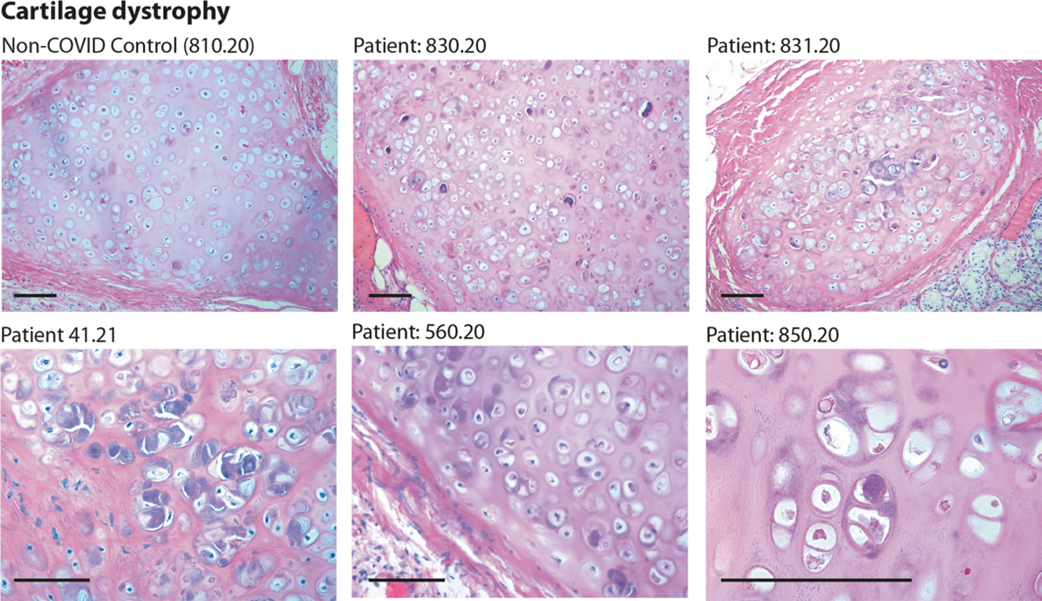
Figure 2
Histology of bronchial cartilage. Haematoxylin and eosin (H&E) staining is shown for five previous COVID-19 patients and one normal control who died from non-COVID pneumonia. In previous COVID-19 patients, chondrocytes appear randomly clustered. Several cells show marked basophilic degeneration, with increased size, dysmorphic nuclear structure, and peripheral halos. In several instances, the chondrocytes have become anucleated. Scale bars: 100 μm.
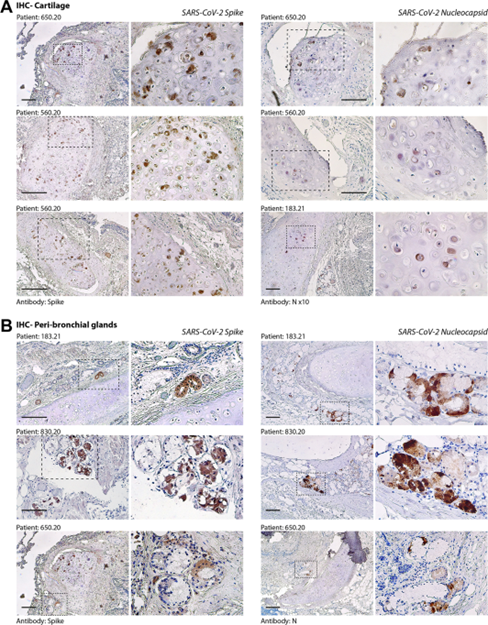
Figure 3
Immunohistochemistry (IHC) for SARS-CoV-2 spike and nucleocapsid (N) antigens. (A) Evidence of SARS-CoV-2 infection in bronchial cartilage chondrocytes. For each of the patients (indicated at the top of each pair of pictures), low- and high-magnification pictures are shown; the magnified area is indicated by a dashed box. Most of the positive chondrocytes (in dark brown) show dysmorphic features. Scale bars in all panels: 100 μm. (B) Same as in panel A for the area surrounding the bronchial structures. Several mucosal glands proximal to the bronchial cartilage were positive for both spike and N antigens.
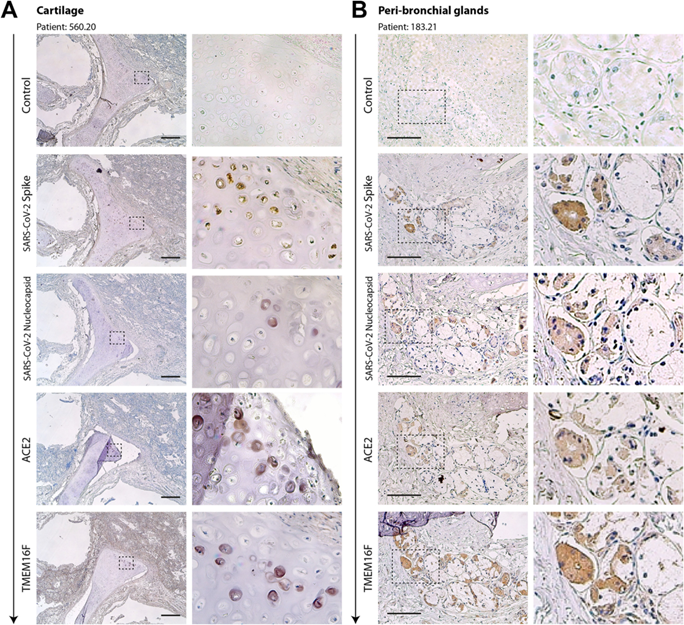
Figure 4
Serial sections of cartilage (A) and the peri-bronchial area (B) of two former COVID-19 patients. Serial sections (from top to bottom, arrow) from the same paraffin block were stained with antibodies against spike, N, ACE2, and TMEM16F, as indicated on the left of each picture. Representative pictures of cartilage and peri-bronchial glands (panels A and B, respectively) are shown. For each picture, low- and high-magnification images are shown; the magnified area is indicated by a dashed box. In most cases, positivity for immunodetection by all four antibodies (brown cells) was in superimposable regions. Scale bars: 100 μm.
ORIGINAL PUBLICATION
Persistent SARS-CoV-2 infection in patients seemingly recovered from COVID-19
Journal of Pathology
Rossana Bussani, Lorena Zentilin, Ricardo Correa, Andrea Colliva, Furio Silvestri, Serena Zacchigna, Chiara Collesi, Mauro Giacca
18 January 2023
Introduction
Two years after the pandemic, it has now become clear that SARS-CoV-2 infection can result in highly heterogeneous disease.
COVID-19 ranges from asymptomatic to deadly, with variable severity according to age, concomitant conditions, and ethnicity [1–3]. Our previous work based on pathological examination of lungs from COVID-19 patients who succumbed to the disease has shown extensive alveolar damage, thrombosis of both micro- and macro-vasculature, and long-term persistence of viral RNA in pneumocytes and endothelial cells [4]. In almost 90% of patients, we detected a large number of dysmorphic pneumocytes, forming syncytial elements as a consequence of the fusogenic activity of the SARS-CoV-2 spike protein [5].
The course of sign and symptom resolution in cases with moderate or severe disease who recover from the acute phase appears equally variegate.
Recovery is prompt and relatively fast in most patients. A recent histopathological examination of elective lung resections to analyse the changes in these COVID-19 survivors identified no differences between the lung parenchyma of these patients to that of controls, in contrast to the acutely infected cases [6]. Epidemiological studies, however, indicate that over one third of patients do not recover completely at 14–21 days post-infection, and some of them remain symptomatic for several months [7–10]. Most patients affected by post-COVID syndrome (commonly called ‘long COVID’) become PCR-negative, indicating apparent elimination of the virus.
Here, we focus our attention on a third category of patients, who apparently clear the virus but nevertheless progress into their disease and eventually die.
We analysed a cohort of consecutive patients who resulted negative at SARS-CoV-2 PCR tests for up to 300 days after remission from acute infection, despite progressive worsening of their clinical status and eventual death. The aim of this study was to study the pathological correlates of this condition, including the possible persistence of viral infection. Post-mortem analyses of these patients revealed the frequent, long-term presence of virus-infected cells in specific lung structures, including bronchial glands and cartilage. Thus, the progressive worsening of clinical conditions in apparently PCR-negative patients after COVID-19 is often associated with the persistent infection of specific cell types in the lung.
Materials and methods, & Results
See the original publication (this is an excerpt version)
Discussion
Here, we analysed a cohort of patients who seemingly recovered from SARS-CoV-2 infection, as concluded from multiple negative PCR tests on both nasopharyngeal swabs and bronchoalveolar lavage protracting for up to 300 days, but who still clinically deteriorated and eventually died.
In most of these cases, pneumonia was the primary or co-primary cause of death, a finding that was confirmed by pathology examination of the lungs. Our work shows that these patients, despite the apparent molecular negativity, still harboured virus-infected cells in their lungs, particularly in the para-bronchial glands and in the bronchial cartilage. This is consistent with the conclusion that these patients had never cleared the infection. The absence of SARS-CoV-2 infection in the respiratory epithelium possibly explains the apparent negativity of these patients to PCR tests performed on bronchoalveolar lavage. These results highlight the relevance of post-mortem pathology examination as a crucial diagnostic tool [26–28].
What is the nature of the virus in the lungs of these individuals who died long after their last PCR-positive test?
Infection appears to be essentially restricted to para-bronchial glands and bronchial chondrocytes, with sporadic presence of virus-infected cells in the vascular wall of medium-sized or large pulmonary arteries, in which clusters of either endothelial cells or mural cells seem to be infected, as shown by the expression of both the spike and the nucleocapsid antigens. Current evidence indicates that the production of significant levels of infectious virus does not necessarily follow the persistence of cells harbouring viral genomes [29, 30]. The lack of infectious virus isolation has been explained by the presence of fragments of the virus RNA and/or the production of defective viral particles [31]. However, irrespective of whether infection is productive or not in terms of new viral particle generation, the virus that we detect in these patients is translationally active, as it generates spike and N antigens that can be detected by antibody-based techniques. Thus, the persistence of these virus-infected cells can induce pathology itself, and also prolong abnormal stimulation of immune reactivity. In particular, the presence of the spike protein on the cell surface could have pathogenic relevance. Our previous work has shown that spike activates the cellular Ca2+-dependent chloride channel and scramblase TMEM16F, thus promoting the externalisation of phosphatidylserine (PS) onto the outer leaflet of the cell plasma membrane and favouring cell–cell fusion [5]. PS externalisation is an important signal for monocyte/macrophage activation (reviewed in [32]) and is essential for the pro-coagulant phase of platelet activation. In platelets, activated TMEM16F itself promotes lipid scrambling [33, 34], and externalised PS serves as an anchoring site for the assembly of the tenase and prothrombinase complex, which jointly enhance the rate of thrombin generation by several orders of magnitude [35]. Our more recent work indeed shows that cells expressing spike on their surface can directly activate TMEM16F in platelets and promote a pro-coagulant phenotype (bioRxiv doi: 10.1101/2021.12.14.472668). Finally, spike-expressing cells also impair the lymphocyte response by inducing fusion of these cells with SARS-CoV-2-infected cells [36]. Thus, the prolonged presence of virus-infected cells in these patients could maintain a pro-inflammatory and pro-thrombogenic status, independent of viral shedding, which might have resulted in the progressive deterioration of lung function and death. Our observations indeed indicate that lung pathology in these patients was essentially no different from that of acutely infected COVID-19 cases.
An important question is to understand whether our findings might have relevance in explaining the persisting symptomatology of individuals with the long COVID syndrome, which consists in either continuous or relapsing and remitting COVID-19 symptoms in PCR-negative subjects [37–40].
Persistent symptoms in long COVID patients have been variously attributed to organ damage, chronic inflammation, autoimmunity, or co-morbidities exacerbated by intervening SARS-CoV-2 infection [41]. Lack of complete clearance of viral infection at >100 days from apparent molecular remission has been documented in the transplanted lungs from an individual who otherwise scored negative to standard PCR tests [42] and in at least another case of an infectious lung transplant in an otherwise PCR-negative donor [43].
There are a few limitations in our study. Given our relatively small sample size, we are not able to identify what are the clinical characteristics of those patients in whom viral clearance had not occurred.
Viral persistence does not seem to correlate with the severity of the prior COVID-19 disease or its duration before the patients became apparently negative, but this will require broader investigation. In situ hybridisation (ISH) for viral RNA would have strengthened our conclusions. However, we found that the post-mortem samples that we had available were of low quality for ISH, with suboptimal RNA preservation (this could also explain the quantitative variation in RNA levels that we measured by qRT-PCR).
Despite these limitations, our findings indicate that SARS-CoV-2 infection can persist significantly longer than suggested by PCR-negative tests on nasopharyngeal swabs or bronchoalveolar lavage fluids.
Whether the persisting infected cells have a pathogenic role in explaining the sequelae of infection in long COVID remains an outstanding question, which deserves further investigation.
Originally published at https://onlinelibrary.wiley.com