The Executive Summary below is a synthesis of the article “Study ties environmental conservation to pandemic prevention”, the 1st article below, published by E&Enews, and the of the article “The costs and benefits of primary prevention of zoonotic pandemics”, published by Science, transcripted below.
Brazilian Scientist Marcia Castro– chair of the Department of Global Health and Population at the Harvard T.H. Chan School of Public Health — participated in the study.
Another Brazilian scientist is Mariana M. Vale, from UFRJ.
Executive Summary
by Joaquim Cardoso MSc
Chief Editor of “The Health Strategist” blog.
February 13, 2022
Prominent policymakers have promoted plans that argue the best ways to address future pandemic catastrophes should entail, “detecting and containing emerging zoonotic threats.” In other words, we should take actions only after humans get sick. The authors of the study sharply disagree.
Reduced deforestation, better management of wildlife trade and hunting, and better surveillance of zoonotic pathogens before they spill over into human populations should be considered “primary pandemic prevention …
The authors explore three practical actions to minimize the impact of future pandemics:
- better surveillance of pathogen spillover
and development of global databases of virus genomics and serology, - better management of wildlife trade, and
- substantial reduction of deforestation.
Those prevention methods would cost $20 billion, according to the authors’ calculations
- … the researchers calculated that the estimated values of lives lost to pandemics is a minimum of $350 billion, with an additional $212 billion in direct economic losses.
- The benefits of “primary prevention,” the experts say, are clear, and go far beyond infectious diseases — e.g.deforestation: “Don’t we all wish we had stopped deforestation in West Africa so we wouldn’t have had HIV
ARTICLE
Study ties environmental conservation to pandemic prevention
E&ENews
By Ariel Wittenberg
02/04/2022
Conservation efforts could be key to preventing the next pandemic, according to a new paper from 20 public health experts around the globe.
Reduced deforestation, better management of wildlife trade and hunting, and better surveillance of zoonotic pathogens before they spill over into human populations should be considered “primary pandemic prevention,” according to the report published in Science Advances, which calculates their annual costs at $20 billion.
Reduced deforestation, better management of wildlife trade and hunting, and better surveillance of zoonotic pathogens before they spill over into human populations should be considered “primary pandemic prevention …
That’s less than 5 percent of the lowest estimated value of lives lost from emerging infectious diseases every year, from the coronavirus to HIV.
In particular, the paper says, current funding for monitoring and surveillance of the wildlife trade is inadequate and enables zoonotic diseases to cross over to humans by increasing human-animal contact.
The authors want increased budgets and personnel for the Convention on International Trade in Endangered Species of Wild Fauna and Flora, the World Organization for Animal Health and national agencies charged with monitoring animal importation.
To prevent deforestation, the authors advocate
- mitigating Amazon deforestation and
- tying conservation measures in smaller forests to investments in strengthening health care systems in those areas.
- The authors also discuss the need for a global viral discovery project to target where prevention activities should be focused and to help identify pathogens when they emerge.
- They also call for more well-trained veterinarians in spillover hotspots to monitor emergent diseases in wildlife and livestock.
Those prevention methods would cost $20 billion, according to the authors’ calculations.
The research then compares the costs of those initiatives to the values of lives lost to every disease since 1918 that originated in animals and went on to kill more than 10 people.
Ultimately, the researchers calculated that the estimated values of lives lost to pandemics is a minimum of $350 billion, with an additional $212 billion in direct economic losses.
Those prevention methods would cost $20 billion, according to the authors’ calculations.
… the researchers calculated that the estimated values of lives lost to pandemics is a minimum of $350 billion, with an additional $212 billion in direct economic losses.
… the estimated values of lives lost to pandemics is a minimum of $350 billion, with an additional $212 billion in direct economic losses.
“By quantifying the benefits of prevention, we are also providing a very strong advocacy,” said Marcia Castro, chair of the Department of Global Health and Population at the Harvard T.H. Chan School of Public Health.
The benefits of “primary prevention,” the experts say, are clear, and go far beyond infectious diseases.
Take the idea of preventing deforestation, for example. Doing so avoids carbon emissions, conserves water supplies, protects Indigenous peoples’ homes and conserves biodiversity.
It also prevents viral spillover into humans, as deforestation, especially in the tropics, brings people into greater contact with wildlife as they enter and clear the areas.
“Don’t we all wish we had stopped deforestation in West Africa so we wouldn’t have had HIV?” asked Stuart Pimm, a professor of conservation ecology at Duke University’s Nicholas School of the Environment.
“The list of things we suggest are things that we know how to do and that have all sorts of benefits — we ought to be protecting forests for other reasons.”
The benefits of “primary prevention,” the experts say, are clear, and go far beyond infectious diseases – e.g.deforestation: “Don’t we all wish we had stopped deforestation in West Africa so we wouldn’t have had HIV
And yet, the authors note, such conservation-related measures to prevent infectious diseases are often ignored by the globe’s top experts working to stop pandemics.
For example, the Global Preparedness Monitoring Board, which is a joint venture of the World Bank and the World Health Organization, wrote a report about the Covid-19 pandemic in September 2020 that pleaded for increased global health security by investing in vaccines, diagnostic tests and pharmaceutical drugs.
It made no mention of preventing zoonotic diseases from spilling over from animals to humans.
Similarly, a panel formed by the Group of 20 nations on “financing the global commons for pandemic preparedness and response” wrote in an April 2021 report that it only considered financing activities after diseases had spilled over.
Though that report did mention the need for global viral surveillance, saying that “the time to the next pandemic may be shorter than many expect,” it did not mention the roles reduced deforestation and better management of wildlife trade and hunting could play in preventing future pandemics.
And yet, the authors note, such conservation-related measures to prevent infectious diseases are often ignored by the globe’s top experts working to stop pandemics
(e.g. (1) Global Preparedness Monitoring Board, (2) a panel formed by the Group of 20 nations on “financing the global commons for pandemic preparedness and response”)
Pediatrician Aaron Bernstein, director of the Center for Climate, Health and the Global Environment at Harvard T.H. Chan School, was the lead author of the Science Advances report out today.
He says the current approach to pandemics that ignores primary prevention methods is nonsensical.
He said the current focus on pharmaceutical advances to prevent the next pandemic “is akin to saying, ‘Let’s just adapt to climate change.’”
“If you said, ‘Let’s not prevent climate change or do any mitigation; let’s just deal with the effects,’ that’s what we are dealing with right now,” he said.
Though global health groups have not advocated for such “primary pandemic prevention” methods, they aren’t entirely novel.
This summer, the Natural Resources Defense Council and the Center for Biological Diversity filed a pair of petitions targeting the Fish and Wildlife Service and the Centers for Disease Control and Prevention asking them to ban imports and exports of live birds and mammals in order to prevent future diseases like Covid-19 from jumping from animals to humans ( Greenwire, Aug. 3, 2021.)
Originally published at https://www.eenews.net on February 4, 2022.
ORIGINAL PUBLICATION

The costs and benefits of primary prevention of zoonotic pandemics
SCIENCE ADVANCES
Aaron S. Bernstein 1 *, Amy W. Ando2,3 , Ted Loch-Temzelides4 ,
Mariana M. Vale5,6 , Binbin V. Li7,8 , Hongying Li9 , Jonah Busch10, Colin A. Chapman11,12,13,14, Margaret Kinnaird15, Katarzyna Nowak16†,
Marcia C. Castro17, Carlos Zambrana-Torrelio9 , Jorge A. Ahumada10, Lingyun Xiao18, Patrick Roehrdanz10, Les Kaufman19, Lee Hannah10, Peter Daszak9 , Stuart L. Pimm8 *, Andrew P. Dobson20,21
4 Feb 2022
Highlights of brazilian authors
Marcia C. Castro17
17Harvard T.H. Chan School of Public Health, Boston, MA 02215, USA.
Mariana M. Vale 5,6
5 Ecology Department, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil.
6 National Institute of Science and Technology in Ecology, Evolution and Biodiversity Conservation, Goiania, Brazil.
Abstract
The lives lost and economic costs of viral zoonotic pandemics have steadily increased over the past century.
Prominent policymakers have promoted plans that argue the best ways to address future pandemic catastrophes should entail, “detecting and containing emerging zoonotic threats.”
In other words, we should take actions only after humans get sick. We sharply disagree.
Humans have extensive contact with wildlife known to harbor vast numbers of viruses, many of which have not yet spilled into humans.
We compute the annualized damages from emerging viral zoonoses. We explore three practical actions to minimize the impact of future pandemics:
- better surveillance of pathogen spillover
and development of global databases of virus genomics and serology, - better management of wildlife trade, and
- substantial reduction of deforestation.
We find that these primary pandemic prevention actions cost less than 1/20th the value of lives lost each year to emerging viral zoonoses and have substantial cobenefits.
LONG VERSION

INTRODUCTION: PREVENTION, NOT JUST CURE
Leaders in public health, medicine, multilateral organizations, global health nonprofits, and many prominent policymakers have promoted plans that argue that the best ways to address future pandemic catastrophes should entail “detecting and containing emerging zoonotic threats (1).”
In other words, we should take actions only after humans get sick. We sharply disagree.
As prominent examples of these approaches that consider solutions only after humans get sick, consider The Global Preparedness Monitoring Board, a joint initiative of the World Bank and the World Health Organization (WHO). This board is tasked with ensuring “preparedness for global health crises.” Its World in Disorder report (September 2020) makes a strong plea to improve global health security that focuses heavily on vaccines, pharmaceuticals, and diagnostic tests (2). Preventing spillover is not mentioned. As another example, the G-20 formed a high-level panel on “Financing the Global Commons for Pandemic Preparedness and Response” tasked with “assessing the current financing systems and suggesting viable solutions for the longer term.” In their progress note of April 2021, the panel clarifies that it only considers financing of post-spillover activities (3).
Much research shows that the spillover of viruses from animals to humans is the major source of pandemic risk (4, 5). The coronavirus disease 2019 (COVID-19) pandemic most likely had its origins in a zoonotic event (6). Hence, the failure to consider minimizing spillover in influential conversations dedicated to preventing the next pandemic perplexes us. These reports hammer on the need to invest more in technology to diagnose, treat, and quickly vaccinate after diseases emerge. If the current pandemic has taught us anything, then it is that no amount of technology can save us from poor governance once an epidemic takes hold in the human population.
Here, we address the need for spillover prevention by evaluating the rate of novel zoonotic virus emergence over the past century. By “novel” we mean previously unknown. We quantify the annualized mortality and economic costs of emerging viruses. We then contrast this with the costs of what we define as primary pandemic prevention actions. We explain the value of better knowledge of viral diversity to primary prevention and then address the three main drivers of pathogen emergence: (i) wildlife trade and hunting, (ii) agricultural intensification and expansion, and (iii) destruction of tropical forests. We examine China’s recent wildlife trade restrictions to reduce spillover risk from wild animal capture and trade. We then illustrate that slowing tropical deforestation is essential to prevention. Last, we note that enhanced wildlife veterinary capabilities are needed to improve spillover surveillance. We conclude that primary prevention costs a fraction of the cost of cures.
ZOONOTIC PANDEMICS ARE FREQUENT AND RISING IN COST
Frequency
The COVID-19 pandemic was predictable but not prevented. Novel viral outbreaks appear at an irregular but increasing rate (Fig. 1 and Table 1). More recent decades have fewer years between outbreaks, fewer years with no outbreaks, and outbreaks that spread to populations on more continents. Earlier work suggested that, over the past century, viruses are detected in humans at a roughly uniform rate of two novel species per year (7). The data illustrated in Fig. 1 show that a higher proportion of these spillover events now gives rise to larger outbreaks. If we express time between outbreaks as cumulative people-years or births, then the rate of pandemic emergence is curiously constant (fig. S1). This result points toward some form of criticality that requires further examination with richer datasets. Nonetheless, it implies that as the number of people alive increases, pandemics will occur more frequently and affect more people.
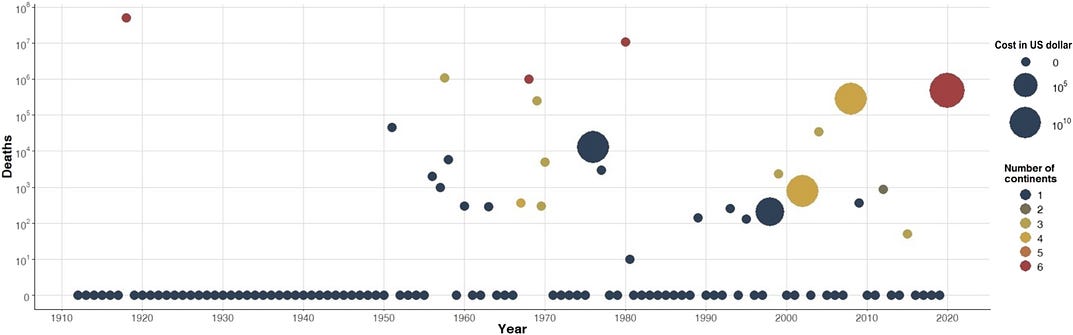
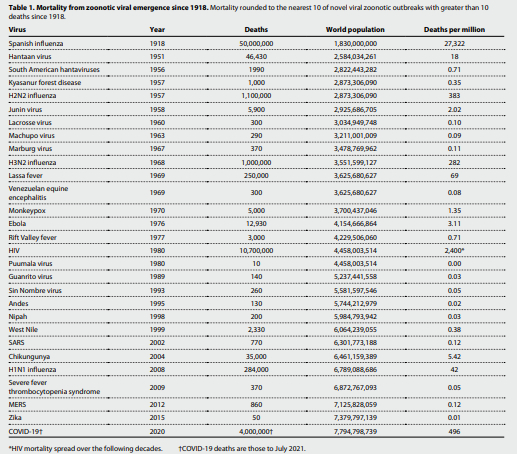
Costs of pandemics
Pandemics have become more frequent and more costly. We previously made preliminary cost estimates for reducing risks of future infectious outbreaks with pandemic potential and compared these to the cost of COVID-19 after its first 6 months (9). Here, we report on a more comprehensive economic approach that estimates the annualized value of lives lost and economic damages for emerging viral zoonoses over the past century. Calculating an annualized cost to viral zoonoses over a long time horizon provides a more robust estimate. It aims to inform policymakers about how much we should spend to prevent spillover each year, rather than an estimate based on a single and outsized pandemic.
To compute how much to spend on preventing spillover, we tabulated every novel viral zoonosis that has appeared since 1918 that killed at least 10 people (Fig. 1 and Table 1). Our core analysis includes Spanish influenza; this improves our ability to calibrate the tail of the distribution composed of severe events that only occur a few times in 100 years. We also present results obtained with that event excluded. Last, we used these data to calibrate a hyperbolic distribution of annual mortality relative to the current world population for novel emerging viral infections. The data provide the frequencies and mean severities of all outbreaks and of severe events. We then use this information to calibrate the remaining parameter of the hyperbolic distribution. See details in the Supplementary Materials.
The baseline expected annual mortality from viral disease epidemics with the current world population is 3.3 million lives.
Estimated willingness to pay (WTP) to prevent mortality can range from $107,000 to $6.4 million per life or more, depending on the country’s wealth (10, 11).
Applying the more conservative range of WTP, we find that avoiding this loss of life translates into a WTP of between $350 billion to $21 trillion annually.
The broad range of values arises because we do not know in which countries future pandemics would occur.
Using the upper range of those WTP values and reducing the likelihood of extreme outbreaks by just 10% cut expected deaths by 300,000 and monetized mortality losses by up to $2 trillion each year (Table 2).
Strategies that curtail the risk of any epidemic by half would save 1.6 million lives a year and reduce mortality costs by $10 trillion.
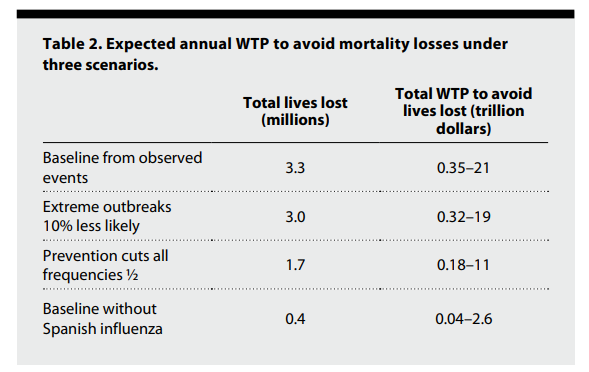
Policymakers and the public may neglect threats from low-probability, future catastrophic pandemics (12). We show the consequences of such neglect by calibrating a distribution of pandemic severity with data that exclude the Spanish influenza event. This oversight leads us to underestimate expected annual lives lost (and the associated costs) by almost an order of magnitude (Table 2, bottom row).
Beyond the WTP for preventing deaths described in Table 2, viral diseases exact direct economic losses that policymakers can use to justify public expenditures. The economic cost of emerging viral zoonoses comes from the lost fraction of world gross national income (GNI) from disease outbreaks of varying severity. Fan et al. (13) calculate the average lost GNI from a pandemic as 0.6% of world GNI. Applying that number to the world GNI of $87 trillion in 2019, the average lost GNI for an outbreak is $522 billion. We have observed 28 outbreaks since 1950, so the expected number of outbreaks of any severity per year is 0.40. Thus, the baseline annual expected loss in GNI from viral zoonotic disease outbreaks is $212 billion. If prevention actions cut those economic losses in half in addition to halving mortality costs, then the additional expected annual savings would be $106 billion. These GNI costs are additional to the WTP costs in Table 2.
In our cost estimates, we excluded major outbreaks of pathogens in domestic livestock or crops. The U.K. foot and mouth epidemic of 2001 cost more than $8 billion, and the emergence of bovine spongiform encephalopathy in Europe in the 1990s had similar financial impact (14, 15). The current outbreaks of swine disease in China and Southeast Asia and the continuing spread of chronic wasting disease in the United States are likewise costly. Each of these livestock pathogens may be only a handful of mutations away from triggering a human pandemic. So, too, are the frequent outbreaks of avian influenza in wild and domestic waterfowl. Emerging pathogens of livestock exploit the same routes to spillover as those that cause human pandemics. The mitigation measures that we describe below to prevent future human pandemics will also benefit livestock disease emergence risks. We can depict the costs graphically from the perspective of the cost during the year a major epidemic occurs and the average cost to prevent it.
The estimates in Table 1 also do not fully quantify the annual damage these viruses cause to human lives or the economy. There is no clear way, for example, to estimate the psychological impact of COVID-19 on people who have lost jobs, relatives, or have had to live in isolation. Nor can we easily ascertain additional costs that stem from medical care deferred because of the pandemic. Such costs may remain hidden for years after a pandemic arises. For example, billions of dollars are spent each year to care for individuals infected with HIV (16).
PRIMARY PREVENTION
The WHO identifies five phases of infectious disease emergence: pre-emergence, emergence, localized transmission, epidemic, and pandemic (17). We recognize spillover as a sixth and critical step in disease emergence (Fig. 2). Viruses spill over into people from wild animals, sometimes by way of domesticated ones (18, 19). Among many causes, greater human and animal contact, livestock rearing, deforestation, and wildlife hunting and trade stand out as drivers of spillover (20).
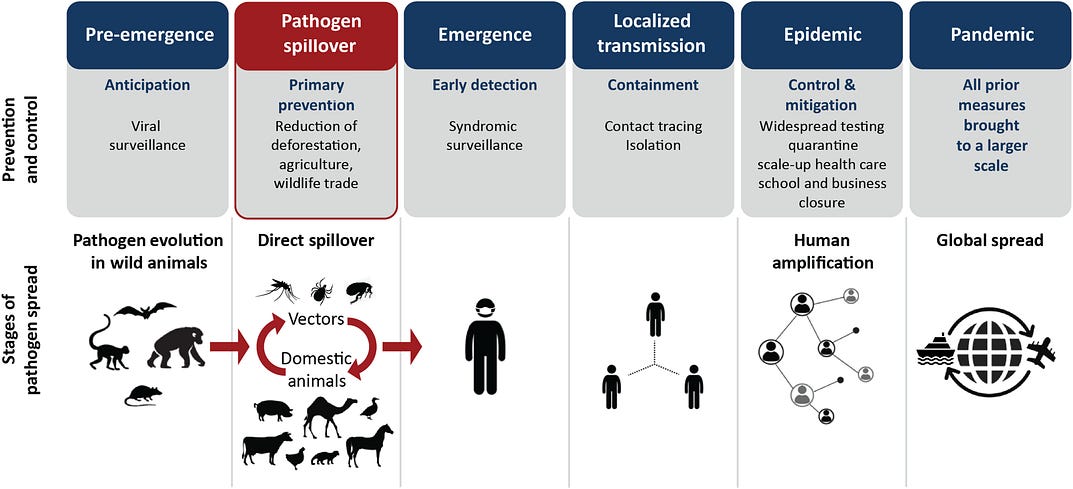
FIG. 2. Phases of pathogen emergence, from local to global.
The World Health Organization identifies five phases to which we have added a sixth: pathogen spillover (in red).
What can we do to minimize the risk of future outbreaks and increase the speed of detecting novel pathogens before they spread locally and globally? The rest of this paper suggests three major courses of action. First, expand viral discovery and surveillance. Second, monitor wildlife hunting and trade as well as large, high-density animal husbandry for viral infections. Last, prevent deforestation and other land-use changes associated with agricultural expansion.
Viral discovery and surveillance: Foundations of primary prevention
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the agent responsible for COVID-19, is a single-stranded RNA virus. So too were all but one of the other pathogens that have caused novel and lethal pandemics over the past 70 years (Table 1). (The exception is monkeypox, a double-stranded DNA virus.) While other infectious pandemics such as cholera, tuberculosis, and a bevy of antimicrobial-resistant organisms remain major health threats, the ability of single-stranded RNA viruses to emerge and produce global upheaval within a year or two is unparalleled.
Even with many actions, including those presented below, taken to prevent viral spillover, some amount of spillover will inevitably occur. When it does, knowing the pathogens that may transfer from an animal to a person and detecting them quickly can foreshorten outbreak containment and inform primary prevention.
Humanity needs a global viral discovery project if we are to prevent future pandemics. An unbiased polymerase chain reaction–based approach targeting viral families [e.g., (16, 17)] could identify the presence of potentially zoonotic pathogens, which may number in the hundreds of thousands (23). In relation to primary prevention, this library would help target where activities should be focused geographically. It would complement further downstream prevention through enabling rapid identification of pathogens when they emerge and accelerating diagnostic test and vaccine development. This pathogen catalog would also benefit livestock and wild animal populations that pathogens threaten.
As an example of the value of viral discovery, we consider Fig. 3 that shows viral accumulation curves for Pteropid bats and macaque monkeys. These curves illustrate the rate at which novel viral pathogens are identified with increasing numbers of animals sampled. They reveal the diversity of viruses to which people who encounter them may be exposed. For Pteropid bats, the data in Fig. 3 suggest that people may be exposed to ~50% of the potential viruses circulating in the wild population if they contact around 400 animals. As 50% represents approximately 30 viruses, this suggests that we have either been lucky not to have had more transmissions or that most viruses cannot replicate in humans. Roughly 50 to 60 viruses circulate in the bats for which we present data. People in the trade are likely exposed to many or all these viruses.
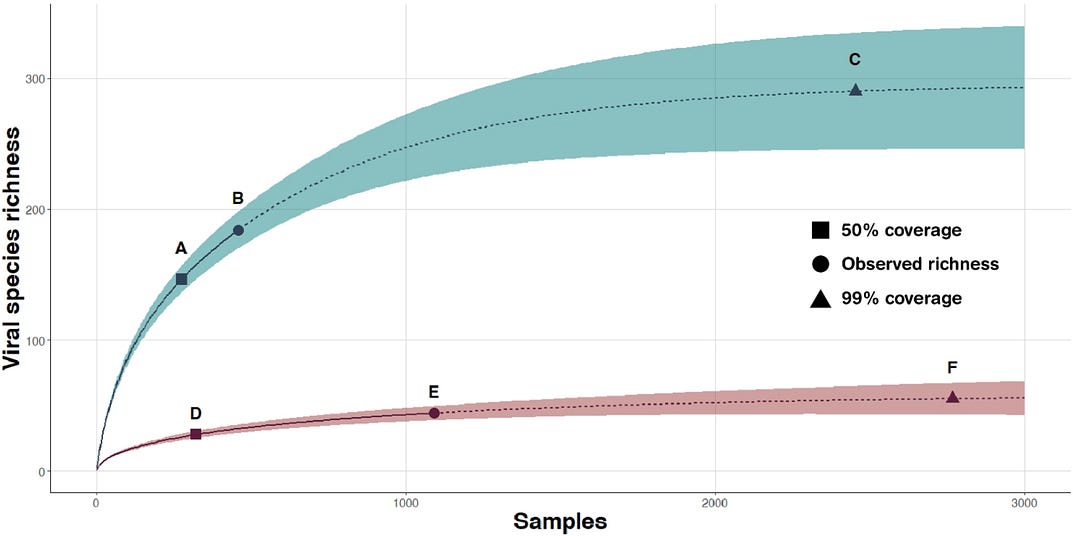
FIG. 3. Viral accumulation curves illustrating the rate at which novel viral pathogens are identified with increasing numbers of animals sampled.
Viral species richness increases for macaque monkeys (blue) and Pteropid bats (red) with the number of animals sampled. Solid lines are from rarefaction; dotted lines are extrapolations (using double sample size). Dots A (samples 310 and richness 141) and D (samples 325 and richness 26) represent 50% sample of sample coverage, and dots C (samples 2325 and richness 284) and F (samples 2705 and richness 52) represent 99% of sample coverage. Dots B and E are the observed viral species richness. Shaded areas represent 95% confidence intervals. Data are from (21, 24).
A caveat for Fig. 3 is that the viruses most frequently encountered — those that form the rising, left side of the accumulation curve — are predominantly those with the highest prevalence in wild host populations. The pathogens detected most frequently are likely to have more efficient transmission and limited virulence. In contrast, rarer viruses will have less efficient transmission, greater virulence, or potentially both. Identifying rare and potentially more virulent viruses will require more extensive sampling of host populations (25, 26). The virulence and transmission efficiency expressed in one host may not correlate to those apparent when the pathogen infects a human or other hosts. Viruses that are relatively harmless in bats, for instance, may be severe in human and other nonvolant mammals (27). As a real-world example, we discuss China’s efforts at pathogen surveillance in the Supplementary Materials.
The value of viral discovery has its limits. Viral genomes cannot be readily used to ascertain host preference or virulence, although there have been recent advances using metagenomic approaches (28). Coupling viral libraries with data from routine serological surveillance of wildlife and livestock farmers, market workers, traders, hunters, wildlife consumers, and other at-risk populations, as well as enhanced surveillance for unusual clusters of symptoms in these groups, would augment the library’s value. A viral genomic library attached to serologic data can give insights into spillover rates and accelerate matching viral genotypes with probable hosts (29). As with the genomes of newly found viruses, the information obtained must be made nonproprietary and available to scientists from all nations to optimize viral identification.
Agriculture and disease emergence
Agricultural intensification and expansion play a major role in pathogen emergence (20, 30). High-density livestock operations can serve as an opportune environment for spillover from wild animals into livestock or as incubators for pandemic influenza strains. Nipah virus emergence in Malaysia occurred on a large pig farm encircled by mango trees and set on the edge of native forests. This arrangement created favorable conditions for spillover of Nipah virus from bats to pigs and from pigs to people (31, 32). Large pig and poultry farms are where the genetic reassortment needed to source pandemic influenza strains may most likely occur (33, 34).
A distinct risk for spillover arises from the farming of wild animals. This practice has grown in the past two decades, and some advocate its use to reduce pressure on wild animal populations (35). With increasing headcounts and proximity to people, wild animal farms represent an emerging spillover risk (36).
Feeding 8 billion people today and many more in the coming decades puts pressure to convert forests and other lands into farms. Conversion of savannahs is also a source of pathogens that we discuss in the Supplementary Materials. Agriculture must be reformed to minimize, or ideally reverse, land conversion (37), and demand for less sustainable food must also be curtailed (38–40).
An analysis of the hundred largest zoonotic outbreaks over the past 30 years points to agricultural intensification as a primary driver of the resurgence of older pathogens such as anthrax, brucellosis, and salmonellosis (41). All the measures that we propose to reduce novel pathogen emergence will also reduce the re-emergence of pathogens that have plagued humans and our domesticated animals for millennia.
The need for more veterinarians
Veterinarians have had a principal role as sentinels for disease emergence. They have been the principal proponents of the One Health concept that integrates human and animal welfare broadly and infectious diseases in particular (42). A country with few veterinarians, many reservoir species, and many people who consume or trade wildlife will be at greater risk for zoonoses. Figure 4 shows the ratio of veterinarians to nonveterinarians against the geographical size of a nation.
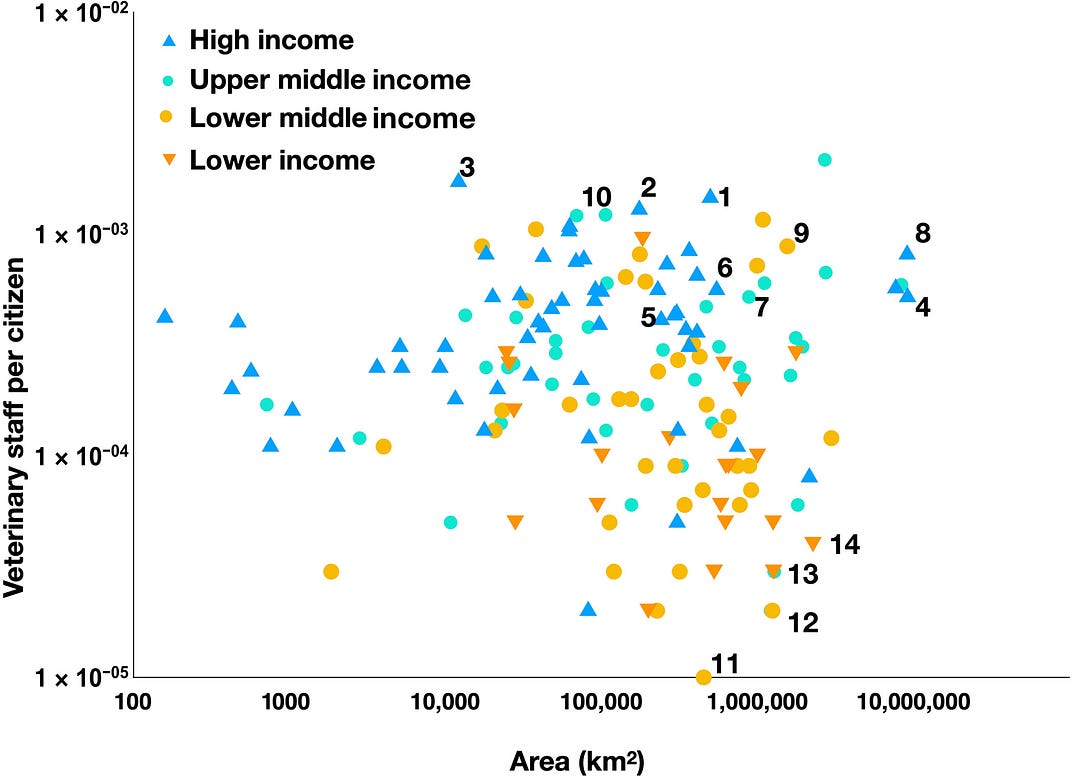
FIG. 4. The national density of veterinarians.
The ratio of veterinarians to civilians plotted against the nation’s area. Countries are color-coded based on World Bank income categories. The text mentions names in bold. Data were absent from the OIE database for several nations, including China and Russia.
Only a small proportion of veterinary workers in any nation work on wildlife diseases and unusual viruses. Most are concerned with domestic livestock and pets. Figure 4 provides a rough view of how easily a virus may slip unnoticed into domestic livestock and then into the human population in places such as Africa, where few veterinarians practice. Southeast Asian countries tend to have more laboratory virologists to examine pathogens that have successfully established in previously uninfected hosts but relatively few people to monitor for pathogen emergence.
Figure 4 demonstrates the national density of veterinarians is independent of the geographical size of a nation. The plot has significant scatter ranging across two orders of magnitude from 2 veterinarians per 100,000 people in many parts of Africa to 2 per 1000 people in Spain (1), Uruguay (2), and the Falkland Islands (3). St. Maarten in the Caribbean has one veterinarian per thousand inhabitants. The United States (4), United Kingdom (5), and France (6) are roughly on a par with Venezuela (7) and not as well-endowed for veterinarians as Canada (8), Mongolia (9), or Cuba (10). Papua New Guinea (11), Angola (12), Peru (13), and South Africa (14) have relatively large land areas and few veterinarians to monitor disease in livestock, letting alone wild animals. More well-trained veterinarians, especially in spillover hotspots, are needed to prevent spillover from wildlife or livestock into people.
Wildlife hunting and trade
The human demand for wild animals also drives pathogen spillover (43). Spillover can occur when people hunt or consume wild animals (44, 45). It can occur at any point in wildlife trade, from the individuals who hunt and capture wild animals to those who consume, wear, or keep wildlife as pets, and everyone in between. Pathogen prevalence in traded animals may grow along the chain of wildlife trade (46). Animals in trade, including wild animals raised in captivity, are often forced into close quarters and unnatural associations with other species (47). These animals may also have higher pathogen prevalence than their wild counterparts (4).
The global scope of wildlife hunting and trade is notable for its breadth and depth. The wildlife trade alone ensnares a quarter of all mammal species, including high percentages of rodents, bats, and primates, which host a high diversity of viral zoonoses (22, 48, 49). The wild animal biomass consumed is also large. In 2010, the annual take of wild animals from the Congo and Amazon basins was between 1.3 million and 4.5 million metric tons, respectively (50). (These are the equivalent weight of 1.8 and 6.2 million cows.) Such capture rates have eradicated entire populations of wildlife species from some countries. For example, in the past 40 years, 12 large vertebrate populations have been extirpated from Vietnam (51). Globally, wildlife hunting pressure threatens more than 300 terrestrial mammal species with extinction (52).
Need for better viral surveillance and data on trade
Data on the species, trade volumes and routes, and long-term trends in legal intranational and international wildlife trade (and certainly the illegal trade) are generally too sparse or unreliable to assess zoonotic disease risk quantitatively (53). Inadequate monitoring and surveillance of wildlife trade enable zoonotic disease emergence. Examples include the spread of the Ebola Reston virus from the Philippines to Maryland, USA via the laboratory animal primate trade (54) and the spread of monkeypox virus from Ghana to Texas through the pet trade in pouched rats (47).
We consider some of the better data available of international wildlife trade in the Supplementary Materials. There, we illustrate the limitations of the present data shortfalls and possible actions to improve surveillance.
Creating institutional capacity for primary prevention in wildlife trade
The world lacks the institutional capacity to monitor wildlife trade for zoonotic disease risk. The Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) is the principal international treaty governing trade in 36,000 plant and animal species listed by the Convention, and 183 countries are parties to it. The secretariat for CITES has stated explicitly that it is not within its mandate to monitor pathogens in the wildlife trade (55). The World Organization for Animal Health (OIE) is perhaps the most closely aligned to this purpose. It conducts rigorous assessments of infectious disease threats to livestock within trades of animals and their products. The OIE has more than 180 member states and authority to list diseases as notifiable, linked to World Trade Organization mandates. Member countries must report annually on the status of a notifiable disease in their country, which measures they are taking to test, control, or eradicate it, and whether they are designating areas as disease-free. Diseases are listed as notifiable primarily if they threaten profits from livestock trade. The OIE also has the authority and capacity to list diseases that threaten wildlife through environmental sources. It rarely uses it. OIE did list amphibian chytridiomycosis, as the disease threatens the trade in amphibians because of its spread in wild populations (56).
A sufficient budget for CITES, OIE, and national agencies charged with monitoring animal importation to conduct the research, monitoring, and enforcement necessary to reduce risky trade could greatly lower spillover risk. More funds alone will not suffice to provide the surveillance needed. Critical personnel to conduct surveillance, such as veterinarians, may be unavailable in many high-risk locations.
Wildlife trade management in China
Past zoonotic disease emergence events informed China’s response to COVID-19. SARS, caused by a bat-borne coronavirus, emerged in China in 2002. Starting in 2003, highly pathogenic avian influenza has emerged and re-emerged in China among waterfowl and poultry, and occasionally among people (34). In 2017, another bat-borne coronavirus spilled into pigs, leading to the death of more than 24,000 piglets in southern China (57). Re-emerging zoonotic diseases including rabies, brucellosis, hemorrhagic fever with renal syndrome, and severe fever with thrombocytopenia syndrome continue to afflict China.
With the advent of COVID-19, China has moved to place greater restrictions on wildlife trade. In January 2020, the Ministry of Agriculture, the State Administration for Market Regulation, and the National Forestry and Grassland Administration issued a temporary ban on all wildlife trade until the end of the epidemic. In February 2020, the Standing Committee of China’s National People’s Congress permanently banned wildlife food consumption to protect health. Food consumption of all terrestrial wildlife is prohibited except for a limited number of farmed species. Loopholes allow wildlife trade for fur, medicine, exhibition, pets, and research (58).
China’s first Biosecurity Law entered into force in April 2021. The law aims to prevent and control infectious diseases and animal and plant epidemics, as well as to promote the development of biotechnology. A revision of the Animal Epidemic Prevention Law released in January 2021 specifies quarantine requirements for farmed wildlife and strengthens wildlife disease surveillance. We consider the wider implications of prevention measures in China and internationally in the Supplementary Materials. We also consider there the costs of reducing China’s consumption of wildlife for food.
Deforestation
Deforestation is arguably the leading driver of pathogen emergence (59–61) and inarguably the greatest threat to terrestrial biodiversity (62, 63). Between 2000 and 2012, 2.3 million km2 of forest were lost globally and the loss in the tropics increased by 3% or 2,101 km2/year (64). Deforestation, particularly in the tropics, brings people into contact with animals as they enter forests to clear them for agriculture or timber, build roads, or work in mines.
Past zoonotic viral disease emergence has been tied to deforestation (65). Models of global spillover risk based on the importance of land cover, especially forest cover, connect to novel virus emergence (59, 66).
Figure S3 (A to E) shows maps of bat, primate, and rodent species richness; tropical and subtropical deforestation; and human population growth (per square kilometer from 2000 to 2020). We map wild bat, primate, and rodent orders as they have unusually high proportions of zoonotic viruses (22, 49). Their diversity is greatest in tropical and subtropical forests, although their patterns differ between regions. These maps illustrate where spillover risk may be possible but not necessarily apparent from past emergence events.
Between 2000 and 2020, as in prior decades, deforestation was most extensive in the Amazon basin, West and Central Africa, and Southeast Asia (fig. S3D). Deforestation creates forest edges that facilitate contact between people and viral reservoir hosts [e.g., (67, 68)]. For example, the detail in fig. S4A shows the deforestation in the Amazon. Linear patterns occur along roads, which also act as foci for further deforestation. Areas without deforestation are often Indigenous-led protected areas (69).
Rapid population growth has occurred in parts of South America, Asia, and Africa (fig. S3E) but does not usually correlate well with deforestation. The juxtaposition of rapid human population growth and deforestation in West Africa likely contributed to the unprecedented scale and location of the 2014 Ebola outbreaks (67).
To explore the interplay of deforestation, population, and host species diversity and to illustrate pathways to prevent spillover near forested regions, the Supplementary Materials discuss two contrasting examples, the Brazilian Amazon and Kibale National Park in Uganda.
Various evidence points to the need to mitigate Amazonian deforestation as a cornerstone of primary pandemic prevention. First, the Amazon is among the world’s most biodiverse regions, particularly for bats and primates (fig. S3, A and B). While people may not eat bats in the Amazon, they commonly hunt primates and large rodents for food (70, 71). Second, since 2012, deforestation in the Brazilian Amazon has risen due to persistent demand for livestock grazing land, with weakening of the country’s forest protection policies and threats to Indigenous stewardship (72). We expect the rise in deforestation there to increase the risk from endemic infectious diseases (73). Third, Amazonian cities have limited capacity to contain infectious epidemics. Last, the Amazon’s connectivity is growing. Flights connect its cities to major population centers in Brazil and abroad, such as Miami in the United States and Panama, which are the crossroads of trade across the Americas and two oceans.
Smaller forests are also important sources of emergent pathogens due to their proximity to densely populated settlements. Kibale National Park is a mere 795 km2 but is one of the few remnant forest patches along the eastern limits of the African equatorial rainforest. Some of Africa’s fastest-growing human populations surround it (fig. S6).
The Amazon example shows that policy improvement, coupled with improved monitoring and enforcement, can be effective at large scales. Countries may achieve robust forest conservation with policy measures similar to the Brazilian Amazon example (74). Recent experiments in Kibale have shown promise in tying conservation to investments in healthcare system strengthening, which the communities living in and around forests may desire (75).
THE COSTS OF PRIMARY PREVENTION
Previously, we provided preliminary estimates of how much primary prevention might cost (9). We presented six estimates of annual costs. We estimated $19 billion to close down China’s wildlife farming industry, based on a Chinese report (76). A total of $476 million to $842 million were needed to reduce spillover from livestock based on (77) and the World Bank One World One Health farm biosecurity intervention program (78). The report provided the cost of implementing enhanced biosecurity for zoonoses around farming systems in low to middle income countries, and we extrapolated those data to the 31 countries with high risk of wildlife viral spillover risk from (65, 66).
The other four were our estimates for viral discovery ($120 million to $340 million), early detection and control ($217 million to $279 million), wildlife trade surveillance ($250 million to $750 million), and programs to reduce spillover from livestock ($476 million to $852 million). The most complicated estimate was reducing deforestation by half ($1.53 billion to $9.59 billion). These broad-brush estimates provide essential insights into the relative magnitude of each task. Here, we provide more details of the underlying issues determining costs and the challenges of implementation.
The costs of viral discovery and spillover surveillance
To compute costs for viral discovery, we chose to use the proposed budget of the Global Virome Project, a decade-long project that seeks to identify 70% of the unknown potentially zoonotic viruses in wildlife globally. It has an estimated budget of $120 million to $340 million per year (23).
To determine the costs of early detection and control, we focused attention on the country surveillance targets of the decade-long United States Agency for International Development (USAID) PREDICT project. The countries were identified due to their high risk of disease emergence from (65, 66) and in Latin America, Africa, South, and Southeast Asia. PREDICT-1 worked in 20 countries for 5 years (Bangladesh, Bolivia, Brazil, Cambodia, Cameroon, China, Democratic Republic of Congo, Gabon, Indonesia, Lao PDR, Malaysia, Mexico, Nepal, Peru, Republic of Congo, Rwanda, Tanzania, Thailand, Uganda, and Vietnam) (79). PREDICT-2 worked in a further 11 countries (Cote d’Ivoire, Egypt, Ethiopia, Ghana, Guinea, Jordan, Kenya, Liberia, Myanmar, Senegal, and Sierra Leone), with minimal work in two others (India and Mongolia) (80). We assumed all programs in this section would need to run in all these 31 high-risk countries.
We identified pilot research projects that successfully identified spillover events for the Nipah virus in Bangladesh (81) and SARS-related coronaviruses in China (82). We analyzed budgets of the cited grant numbers in these papers by searching the U.S. National Institutes of Health database (83) and estimated the amount spent on surveillance in the field. To maximize the likelihood of early detection of small numbers of spillover cases, we estimated that these programs would need to be scaled up by an order of magnitude. We based this scaling on the three Nipah virus spillover events identified in Bangladesh by Nikolay et al. (81) and the geographical coverage of the “Nipah belt” that this project funded for syndromic hospital surveillance. We used the published budgets in the request for proposal document for National Institute of Allergy and Infectious Diseases Centers for Research in Emerging Infectious Diseases (NAIAD CREID) contracts (previously called “Emerging Infectious Disease Research Centers”). These are designed specifically to identify early spillover in emerging disease hotspot countries (84).We then estimated the cost of control programs for these early outbreaks to include testing, isolation, and quarantine of small numbers of cases to reduce stransmission based on costs from the budgets that funded (81), available in (83), and of partial budgets allocated for (84):
1) Pilot projects ($500 thousand to $700 thousand per year, 10 per country for 31 countries) = $155 million to $217 million.
2) NIAID CREID contracts ($1.5 million per year, for 31 countries) = $46.5 million per year.
3) Isolation and quarantine ($500 thousand per year, for 31 countries) = $15.5 million per year.
Summing these three programs, the total cost of early detection and control programs for the 31 high-risk countries would be between $217 million to $279 million per year.
The costs of monitoring and managing wildlife trade
Our estimate of monitoring wildlife trade had a relatively large range ($250 million to $750 million) because of the considerable complexities of expanding existing programs, which we now explore.
We suggest expanding the OIE’s scope to achieve a more holistic approach to managing disease emergence from wildlife trade. This is consistent with recommendations put forward by the OIE itself in early 2021 (85). To do this will require more resources. The annual 2018 operating budget of the OIE was $35 million. Substantially increasing this budget should provide resources sufficient to drive a globally significant disruption of this pathway for disease emergence. The costs of surveillance could be covered by governments or passed to the wildlife trade businesses (e.g., fashion houses, pet, and aquarium sellers) and consumers, with traders requiring permits before import. Permits are already necessary for CITES-listed species. This must be the new cost of doing business in a world that must now live with COVID-19.
While CITES may not be well-positioned to address pathogen risk in wildlife trade, wildlife enforcement networks may. They are underfunded for this task. The ASEAN Wildlife Enforcement Network (WEN) is the longest standing. It launched on 1 December 2005, with 10 member countries, and has an annual budget of some $30,000 (86).
The current annual budgets of all WENs are low and insufficient to execute their missions. The U.S. State Department has been the primary supporter of WENs. Its funds channel through nongovernmental organizations (NGOs), such as TRAFFIC and WildAid. They have budgets of $17.4 million and $10.4 million (87, 88), respectively. The U.S. State Department has been the sole supporter of the Central American and Dominican Republic Wildlife Enforcement Network (CAWEN or ROAVIS in Spanish). It provides additional support to related counter-trafficking of wild flora and fauna.
The Wildlife Trafficking, Response, Assessment and Priority Setting Project (TRAPS), financed by USAID and implemented by TRAFFIC and the International Union for Conservation of Nature (IUCN), identifies and advances interventions to break trafficking chains and disrupt organized criminal trade networks (89). Reducing Opportunities for Unlawful Transport of Endangered Species is a sister program to TRAPS and provides data analytics to support the transportation sector in battling illegal wildlife trade (90).
Other wildlife conservation networks supported by NGOs or countries offer similar opportunities to monitor zoonotic disease emergence from the wildlife trade. For example, “Red Jaguar” is supported by the Europe–Latin America Technical Assistance Programme against Transnational Organized Crime (El PAcCTO). It seeks to combat environmental crimes, including wildlife trafficking in Latin America. The U.S. Department of Interior’s International Technical Assistance Program can extend its support for this effort, which closely aligns with WENs. It promotes more broadly based wildlife conservation and disease surveillance. Funding to support WENs and other transboundary law enforcement efforts is crucial to building the capacity to respond effectively to spillover risk from international wildlife trafficking.
As a major player in global wildlife trade, the United States has an incentive to lead the development of shared objectives and, ultimately, regional funding mechanisms for the self-sufficiency of WENs. They need between $0.5 million to $1 million per year to operate effectively. That sum is more than 20 times the amount ASEAN has had (86). The CITES Secretariat has the standing and international reach to advance WENs zoonotic spillover prevention measures as part of WENs’ trade monitoring protocols. These measures should be buttressed through coordination with OIE, as would be consistent with the CITES-OIE memorandum of understanding.
The costs of managing landscapes and protecting forests
In the Supplementary Materials, we map out the species richness of bats, primates, and rodents — the three taxa most likely to cause viral spillover. More than two-thirds of all known species live between 30oN and 30oS. We also show the past two decades of deforestation and human population increase. Bats are most diverse in the Amazon, primates in the Congo, and rodents have major centers of diversity in South America, Africa, and Southeast Asia. Roads deep into the Amazon created extensive edge areas bringing people into contact with exceptionally diverse vertebrate communities. In West and Central Africa, rapid human population growth into previously forested areas spurred wild animal meat consumption and the various HIV spillovers.
Previously, we used a broad range of evidence-based costs associated with preventing deforestation to estimate that cutting deforestation by half in emerging infection hotspots would cost between $1.53 billion and $9.59 billion per year (9). Using the low-end cost model estimates, 50% reduction of deforestation in the 10% of the tropics that are emerging infection hotspots and 34% reduction of deforestation in other tropical forests carry an annual cost of $3.23 billion (2020 USD) (91). The Supplementary Materials consider the costs of slowing deforestation for the Amazon, where population densities are low and for Kibale National Park, next to one of Africa’s most rapidly expanding populations.
Several policies enabled better protection of the Amazon. These policies expanded protected areas, recognized Indigenous territories, put market restrictions on illegal landholdings, placed credit restrictions on municipalities with high deforestation rates, and created payment for ecosystem service programs benefiting small farmers (92, 93). State-of-the-art science satellite monitoring and improved enforcement of existing laws buttressed these policies (92).
These actions to curtail deforestation cost the Brazilian government $1 billion per year (~0.1% of Brazil’s total federal budget), primarily not only from federal funds but also with contributions from state and cities (93). An Amazon Fund, including a $1-billion commitment from Norway between 2009 and 2019, supported actions to reduce deforestation (92).
Reductions in deforestation for the sparsely populated and relatively intact Amazon were approximately $650 spent per hectare saved during 2005–2012 or roughly $93 per hectare per year on average. In contrast, thousands of dollars per hectare would pay the full opportunity cost to maintain privately held forest.
Conservation investments in more densely populated and fragmented forests differ. In one such place, Kibale National Park, Uganda, costs for community health system strengthening, education, law enforcement, and general park operations sum to $33 per hectare (estimated based on a compilation by authors of expenditures for programs in Kibale; data are available on request). When we apply this to the approximately 1,032,000 km2 of forest in emerging infection hotspots worldwide (66, 91), the sum is $3.3 billion per year.
DISCUSSION
Costs and benefits
Here, we estimate the annualized economic and health costs of viral zoonotic emergence and provide primary prevention activities and capacity building estimates substantially refined from prior work. We find that the sum of our median cost estimates of primary prevention (~$20 billion) are ~1/20 of the low-end annualized value of lives lost to emerging viral zoonoses and <1/10 of the annualized economic losses.
Our estimates of annualized WTP for the primary prevention of viral zoonoses depend heavily on severe events such as COVID-19, HIV, and Spanish influenza. Countervailing forces bear upon pandemic risk. Risks will fall with advances in technology that enable more rapid diagnostic tests, vaccines, and medications for newly emergent diseases. The efficacy of these advances depends on expanding viral surveillance in ways that increase our ability to develop tests and vaccines rapidly and deploy them widely. In addition, investments in strengthening health care systems may substantially reduce the disease burdens that exact heavy human and economic tolls in much of the world (94). They may also enhance the ability to detect and monitor disease outbreaks. At the same time, more people are living in densely populated cities, global travel has proliferated, and governance is unstable in many countries. All of these can increase the risk of disease spread and impact. More research dedicated to understanding how urbanization, global travel, and contact with more remote communities may alter the risk of pathogen spread would better inform potential damages from disease emergence.
We underestimate the economic and health costs of emerging viral zoonoses as we have omitted multiple causes of indirect damage. The estimates in Table 2 do not include costs from, as examples, (i) morbidity, including, e.g., the psychological harms that result from lost jobs, lost relatives, or social isolation; (ii) delayed medical treatments; or (iii) loss or delays of education. In short, the WTP for preventing death (in the value of a statistical life) from an emerging virus captures only a fraction of the value that may come from primary prevention activities.
The distinction between primary prevention and those actions taken after emergence has occurred is not semantic. The former creates a broad sweep of benefits, while the latter tends to affect a single disease. Most obviously, a vaccine can be effective at reducing the prevalence of a single, currently circulating, infectious disease, but it can never prevent the emergence of novel pathogens.
Consider preventing deforestation. It avoids carbon emissions, conserves water supplies, protects Indigenous Peoples’ rights, conserves biodiversity, and suppresses the emergence of novel and well-known pathogens (95). Many of these values, especially emerging infectious disease risk abatement, are poorly understood and merit further scientific inquiry. Lacking a greater understanding of these values limits optimizing investments and decision-making to protect health and nature (96). Yet, considering the relatively better-known values — such as for carbon sequestration — the benefits of protecting forests are potentially massive, independent of any effect on pandemic risk reduction (9).
Furthermore, while growing urban populations and more frequent global travel amplify pandemic risks, the root cause of viral pandemics lies in a pathogen’s movement from an animal to a person. No amount of travel restriction, nor surveillance, nor outmigration from cities is likely to prevent spillover.
In addition, the viral prospecting that forms a significant component of spillover prevention will concomitantly speed the development of tests and vaccines that will be essential components of control once spillover has occurred. Recent studies point to powerful new methods of identifying and prioritizing potential human infecting viruses from their genome sequences (97). Such advances would massively increase the cost efficiency of the Global Virome databases described here.
The case for prevention
We propose primary prevention actions and recommendations for their implementation as a blueprint for decision-makers to forestall the next viral pandemic. Our estimates of their cost-effectiveness would benefit from greater certainty as to how great a reduction of viral zoonotic disease emergence events would be achieved were they implemented. Notwithstanding this, the orders of magnitude difference in costs between primary prevention actions and actions that work to control epidemics and pandemics make even small effects worthwhile. Even a 1% reduction in risk of viral zoonotic disease emergence would be cost-effective.
While each of the actions that we propose can reduce the potential threat of future pandemics, no single intervention will prevent a pandemic. One must view these interventions as complementary wedges akin to those proposed to slow and reverse climate change and biodiversity loss (40, 98). Their implementation can create a significant number of jobs across a range of skills as the global economy reconfigures in the wake of the pandemic.
The health, societal, and economic shocks from the COVID-19 pandemic compel consideration of preventing similar future pandemic disasters. To date, most money has been spent after viruses reach epidemic or pandemic scale, and their economic and health damages have grown immensely. Monothetic “magic bullets,” including diagnostic tests, treatments, and vaccines, failed to control COVID-19 as it spread around the globe and exacted the largest health and economic toll of any pathogen in recent history. This makes plain that we cannot solely rely upon post-spillover strategies to prevent a similar fate in the future.
We argue that substantial gaps in knowledge, institutional capacity, and financial resources limit the ability to avert pathogen emergence. We recommend scientific inquiry, policy actions, and financial and organizational resources needed to forestall the next pandemic and estimate that primary pandemic prevention actions are remarkably inexpensive compared to the many lives emerging viral zoonoses take or the direct economic damage they cause. The findings and recommendations of this paper bear upon recommendations that will emerge from the Convention on Biological Diversity’s 15th Conference of the Parties as well as ongoing, high-level meetings to determine the most prudent paths forward to address pandemic risk and climate change.
References and additional information
See the original paper
About the authors & affiliations
Aaron S. Bernstein1 *, Amy W. Ando2,3 , Ted Loch-Temzelides4 , Mariana M. Vale5,6 , Binbin V. Li7,8 , Hongying Li9 , Jonah Busch10, Colin A. Chapman11,12,13,14, Margaret Kinnaird15, Katarzyna Nowak16†, Marcia C. Castro17, Carlos Zambrana-Torrelio9 , Jorge A. Ahumada10, Lingyun Xiao18, Patrick Roehrdanz10, Les Kaufman19, Lee Hannah10, Peter Daszak9 , Stuart L. Pimm8 *, Andrew P. Dobson20,21
1 Boston Children’s Hospital and the Center for Climate, Health and the Global Environment, Boston, MA 02115, USA.
2 Department of Agricultural and Consumer Economics, University of Illinois Urbana-Champaign, Champaign, IL 61801, USA.
3 Resources for the Future, 1616 P Street NW, Washington, DC 20036, USA.
4 Department of Economics and Baker Institute for Public Policy, Rice University, Houston, TX 77005, USA.
5 Ecology Department, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil.
6 National Institute of Science and Technology in Ecology, Evolution and Biodiversity Conservation, Goiania, Brazil.
7 Environment Research Center, Duke Kunshan University, Kunshan, Jiangsu Province 215317, China.
8 Nicholas School of the Environment, Duke University, Durham, NC 27708, USA.
9 EcoHealth Alliance, 520 Eighth Avenue, New York, NY 10018, USA.
10Moore Center for Science, Conservation International, Arlington, VA 22202, USA.
11Wilson Center, 1300 Pennsylvania Avenue NW, Washington, DC 20004, USA.
12Center for the Advanced Study of Human Paleobiology, George Washington University, Washington, DC 20004, USA.
13School of Life Sciences, University of KwaZulu-Natal, Pietermaritzburg, South Africa.
14Shaanxi Key Laboratory for Animal Conservation, Northwest University, Xi’an, China. 15Practice Leader, Wildlife, WWF International, The Mvuli, Mvuli Road, Westlands, Kenya.
16The Safina Center, 80 North Country Road, Setauket, NY 11733, USA.
17Harvard T.H. Chan School of Public Health, Boston, MA 02215, USA.
18Department of Health and Environmental Sciences, Xi’an Jiaotong-Liverpool University, Suzhou, Jiangsu Province 215123, China.
19Department of Biology and Pardee Center for the Study of the LongerRange Future, Boston University, Boston, MA 02215, USA.
20Department of Ecology and Evolutionary Biology, Princeton University, Princeton, NJ 08544, USA.
21Santa Fe Institute, Hyde Park Road, Santa Fe, NM 87501, USA.
Originally published at https://www.science.org