health and tech
institute
Joaquim Cardoso MSc
Founder, Chief Researcher & Editor
February 9, 2023
ABSTRACT
BACKGROUND
- The efficacy of a single dose of pegylated interferon lambda in preventing clinical events among outpatients with acute symptomatic coronavirus disease 2019 (Covid-19) is unclear.
METHODS
- We conducted a randomized, controlled, adaptive platform trial involving predominantly vaccinated adults with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in Brazil and Canada.
- Outpatients who presented with an acute clinical condition consistent with Covid-19 within 7 days after the onset of symptoms received either pegylated interferon lambda (single subcutaneous injection, 180 μg) or placebo (single injection or oral).
- The primary composite outcome was hospitalization (or transfer to a tertiary hospital) or an emergency department visit (observation for >6 hours) due to Covid-19 within 28 days after randomization.
RESULTS
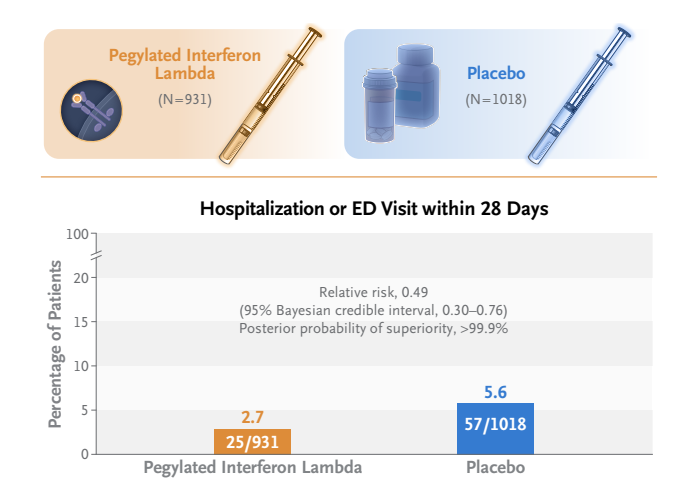
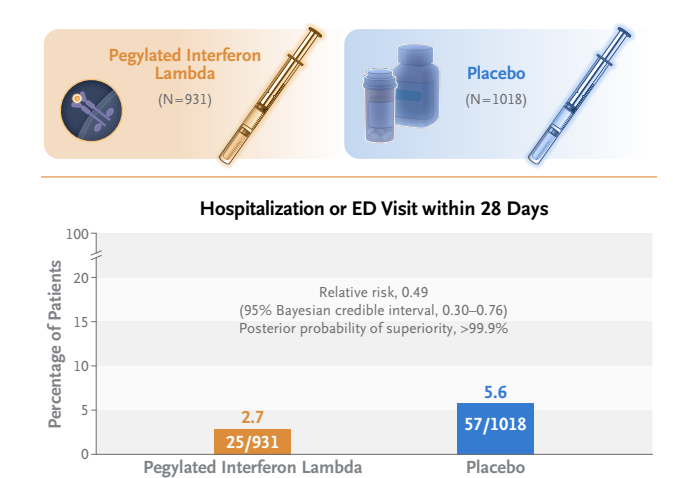
- A total of 933 patients were assigned to receive pegylated interferon lambda (2 were subsequently excluded owing to protocol deviations) and 1018 were assigned to receive placebo.
- Overall, 83% of the patients had been vaccinated, and during the trial, multiple SARS-CoV-2 variants had emerged.
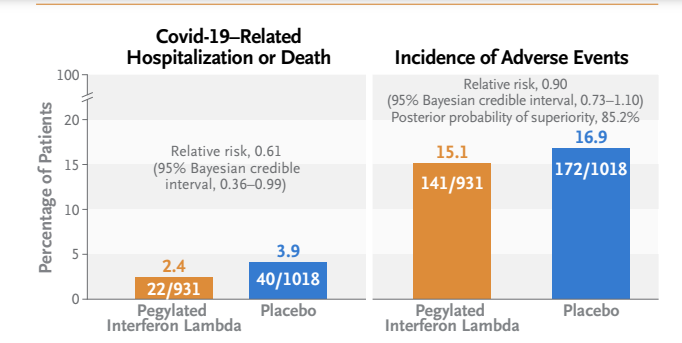
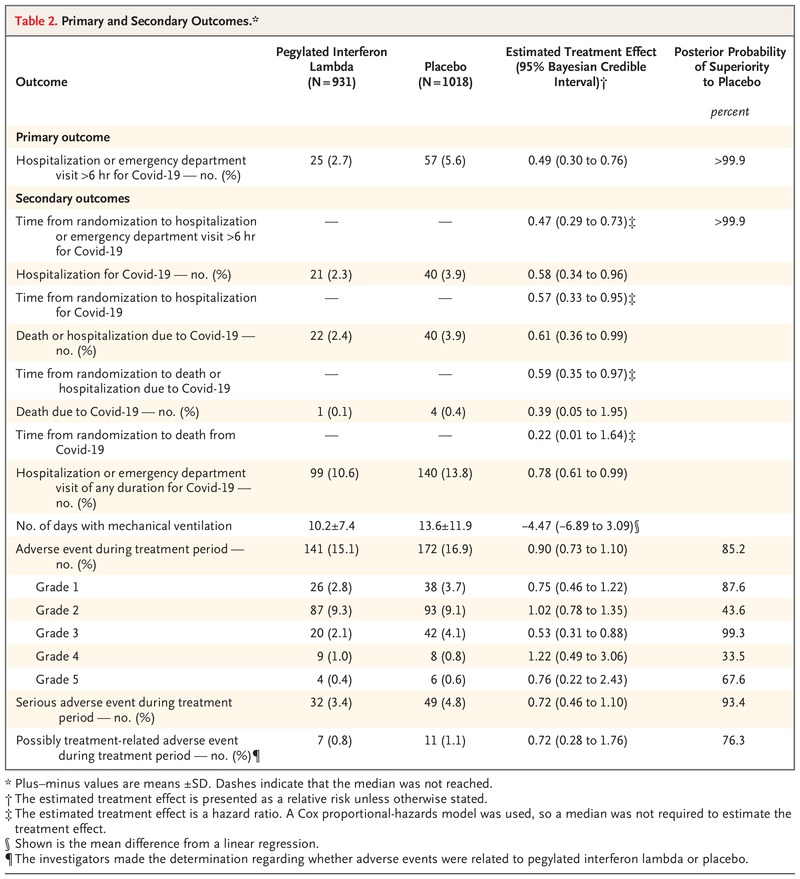
- A total of 25 of 931 patients (2.7%) in the interferon group had a primary-outcome event, as compared with 57 of 1018 (5.6%) in the placebo group, a difference of 51% (relative risk, 0.49; 95% Bayesian credible interval, 0.30 to 0.76; posterior probability of superiority to placebo, >99.9%).
- Results were generally consistent in analyses of secondary outcomes, including time to hospitalization for Covid-19 (hazard ratio, 0.57; 95% Bayesian credible interval, 0.33 to 0.95) and Covid-19–related hospitalization or death (hazard ratio, 0.59; 95% Bayesian credible interval, 0.35 to 0.97).
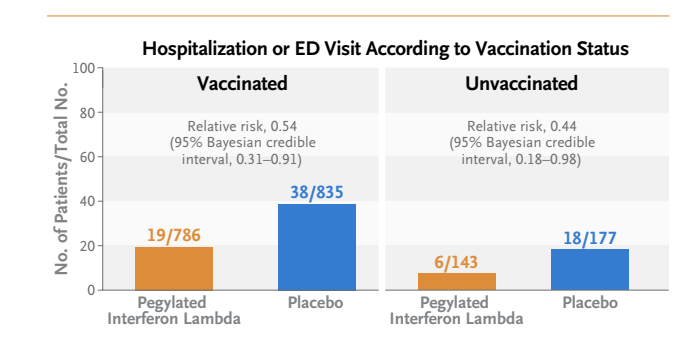
- The effects were consistent across dominant variants and independent of vaccination status.
- Among patients with a high viral load at baseline, those who received pegylated interferon lambda had lower viral loads by day 7 than those who received placebo.
- The incidence of adverse events was similar in the two groups.
CONCLUSIONS
- Among predominantly vaccinated outpatients with Covid-19, the incidence of hospitalization or an emergency department visit (observation for >6 hours) was significantly lower among those who received a single dose of pegylated interferon lambda than among those who received placebo.
(Funded by FastGrants and others; TOGETHER ClinicalTrials.gov number, NCT04727424. opens in new tab.)
INFOGRAPHIC

Early Treatment with Pegylated Interferon Lambda for Covid-19
NEJM
Gilmar Reis, M.D., Ph.D., Eduardo A.S. Moreira Silva, M.D., Ph.D., Daniela C. Medeiros Silva, M.D., Ph.D., Lehana Thabane, Ph.D., Vitoria H.S. Campos, Thiago S. Ferreira, M.D., Castilho V.Q. Santos, Ana M.R. Nogueira, M.D., Ana P.F.G. Almeida, M.D., Leonardo C.M. Savassi, M.D., Ph.D., Adhemar D. Figueiredo-Neto, M.D., Ph.D., Ana C.F. Dias, Ph.D., et al., for the TOGETHER Investigators*
Introduction
The identification of convenient, widely available, and effective antiviral therapies against coronavirus disease 2019 (Covid-19) for outpatients is of great importance. In infected cells, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) induces weak expression of naturally produced type III interferons, which are an early line of defense in upper respiratory tract infections.1 Treatment with an exogenous source of interferon lambda such as pegylated interferon lambda could stimulate antiviral immunity and treat early SARS-CoV-2 infection.2
In more than 20 clinical trials, the administration of pegylated interferon lambda to more than 4000 patients for a variety of conditions (including hepatitis B, C, and D, and Covid-19) has provided a well-understood safety and side-effect profile.3,4 Pegylated interferon lambda has broad-spectrum antiviral activity in numerous cell cultures, animal models, and clinical settings.3–8 With respect to SARS-CoV-2, pegylated interferon lambda has potent in vitro and in vivo activity.3,9 The results of two phase 2 studies characterizing the effect of pegylated interferon lambda on SARS-CoV-2 viral load have been published.10,11 To further evaluate the effect of this agent on clinical outcomes, we used the TOGETHER trial master protocol12 to conduct a large, phase 3, randomized, placebo-controlled adaptive platform trial involving outpatients with Covid-19 in Brazil and Canada.
Methods & Results
See the original publication (this is an excerpt version)
Discussion
This phase 3 trial, which was conducted in a predominantly vaccinated population infected with various SARS-CoV-2 variants of concern, showed the efficacy of a single subcutaneous dose of pegylated interferon lambda administered within 7 days after the onset of symptoms (mean, 3 days). This regimen resulted in a greater than 50% reduction in the risk of a primary-outcome event. Our trial findings were consistent across the SARS-CoV-2 variants of concern and across multiple subgroups according to vaccination status.
In order to place the findings in the context of available oral therapies for outpatient treatments, we examined the effect of pegylated interferon lambda on the composite outcome of Covid-19–related hospitalization or death. Our trial showed a 41% reduction in time to death or hospitalization due to Covid-19 (hazard ratio, 0.59; 95% Bayesian credible interval, 0.35 to 0.97). Among patients who had begun to receive interferon within 3 days after symptom onset, this reduction increased to 65% (relative risk, 0.42; 95% Bayesian credible interval, 0.21 to 0.80).
During the conduct of this trial, two periods of disruption in the supply chain for drugs occurred. This was a multigroup trial, so we included only a concomitant control group in our analysis.18 Since the completion of our trial, a polymorphism in the innate antiviral response gene OAS1 has been associated with clearance of SARS-CoV-2, and a common haplotype could be used to identify patients with an increased likelihood of response.19 Evaluation of the prevalence of this haplotype is warranted.
In this trial involving largely vaccinated outpatients who presented with acute symptomatic Covid-19, the incidence of hospitalization or an emergency department visit due to Covid-19 was significantly lower among patients who received a single dose of pegylated interferon lambda than among those who received placebo. These results, which were observed regardless of viral variant, offer the possibility that a single-dose regimen can play a role in the response to Covid-19.
About the authors & affiliations
Gilmar Reis, M.D., Ph.D., Eduardo A.S. Moreira Silva, M.D., Ph.D., Daniela C. Medeiros Silva, M.D., Ph.D., Lehana Thabane, Ph.D., Vitoria H.S. Campos, Thiago S. Ferreira, M.D., Castilho V.Q. Santos, Ana M.R. Nogueira, M.D., Ana P.F.G. Almeida, M.D., Leonardo C.M. Savassi, M.D., Ph.D., Adhemar D. Figueiredo-Neto, M.D., Ph.D., Ana C.F. Dias, Ph.D., Adelino M. Freire Júnior, M.D., Ph.D., Carina Bitarães, R.N., Aline C. Milagres, R.N., Eduardo D. Callegari, M.D., Maria I.C. Simplicio, B.Sc.Pharm., Luciene B. Ribeiro, R.N., M.P.H., Rosemary Oliveira, Ofir Harari, Ph.D., Lindsay A. Wilson, M.Sc., Jamie I. Forrest, Ph.D., M.P.H., Hinda Ruton, M.Sc., Sheila Sprague, Ph.D., Paula McKay, M.Sc., Christina M. Guo, B.Com., Eve H. Limbrick-Oldfield, Ph.D., Steve Kanters, Ph.D., Gordon H. Guyatt, M.D., Craig R. Rayner, Pharm.D., Christopher Kandel, M.D., Mia J. Biondi, Ph.D., Robert Kozak, Ph.D., Bettina Hansen, Ph.D., M. Atif Zahoor, Ph.D., Paul Arora, Ph.D., Colin Hislop, M.B., B.S., Ingrid Choong, Ph.D., Jordan J. Feld, M.D., M.P.H., Edward J. Mills, Ph.D., F.R.C.P., and Jeffrey S. Glenn, M.D., Ph.D. for the TOGETHER Investigators*
The authors’ affiliations are as follows:
ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto —
all in California;
the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.),
the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and
Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the
Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.),
the Public Health Care Division, Ibirité (C.B., A.C.M.),
the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.),
the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and
the Public Health Care Division, Brumadinho (E.D.C., B.H.) —
all in Brazil;
the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto —
all in Canada;
Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam,
the Netherlands (B.H.).