The Lancet
Prof Ildiko Lingvay, MD; Priya Sumithran, PhD; Ricardo V Cohen, MD; Prof Carel W le Roux, MD
September 30, 2021
Summary
- Obesity is now recognised as a disease that is associated with serious morbidity and increased mortality.
- One of its main metabolic complications is type 2 diabetes, as the two conditions share key pathophysiological mechanisms.
- Weight loss is known to reverse the underlying metabolic abnormalities of type 2 diabetes and, as such, improve glucose control;
loss of 15% or more of bodyweight can have a disease-modifying effect in people with type 2 diabetes, an outcome that is not attainable by any other glucose-lowering intervention.
- Furthermore, weight loss in this population exerts benefits that extend beyond glycaemic control to improve risk factors for cardiometabolic disease and quality of life.
We review the evidence supporting the role of weight loss in the management of type 2 diabetes and propose that many patients with type 2 diabetes would benefit from having a primary weight-centric approach to diabetes treatment.
We discuss the logistical challenges to implementing a new weight-centric primary treatment goal in people with type 2 diabetes.
We review the evidence supporting the role of weight loss in the management of type 2 diabetes and
propose that many patients with type 2 diabetes would benefit from having a primary weight-centric approach to diabetes treatment.
Conclusions (copied from the end of the paper)
- The time is right to consider the addition of substantial (ie, double-digit) weight loss as a principal target for the treatment of many patients with type 2 diabetes.
This approach would
- address the pathophysiology of the disease process for type 2 diabetes;
- recognise adipose tissue pathology as a key underlying driver of the continuum of obesity, type 2 diabetes, and cardiovascular disease;
- and reap metabolic benefits far beyond glycaemia.
Such a change in treatment goals would recognise obesity as a disease with reversible complications and require a shift in clinical care.
LONG VERSION
Introduction
Over the past decade, management of patients with type 2 diabetes has undergone a major conceptual change, with treatment objectives shifting to include a cardiocentric goal in the subpopulation with high cardiovascular risk, alongside the singular glucocentric goal that has long been held.1
This advance was driven by studies showing that several glucose-lowering agents, used in addition to standard of care, further lower the risk of myocardial infarction, stroke, and cardiovascular death,2,3 largely independently of lowering blood glucose concentration. 4 , 5
Yet, even after this landmark evolution, the treatment framework for type 2 diabetes is primarily focused on preventing or treating the downstream metabolic consequences, which tend to occur late in the disease course.
A promising opportunity lies in intervening upstream to address the key pathophysiological driver of type 2 diabetes and its associated metabolic complications: obesity (figure 1). Sustained loss of at least 15% bodyweight has a major effect on progression of type 2 diabetes, inducing remission in a large proportion of patients and markedly improving metabolic status in many others. 6 , 7
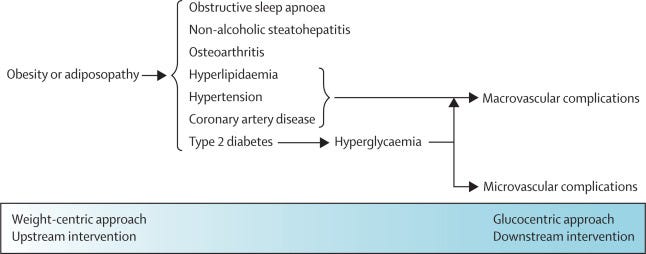
Figure 1 Illustration of the wide-ranging benefits of an upstream weight-centric approach versus a glucocentric management approach
Until 2021, the only intervention that could routinely result in maintenance of weight loss of this magnitude was bariatric surgery. However, despite its considerable benefits, a complex surgical procedure is not feasible or scalable as the mainstay for a population-wide intervention. Now, with effective pharmaceuticals to reduce bodyweight in the pipeline, many of which can also directly lower blood glucose concentration, it is time to rethink treatment goals for patients with type 2 diabetes to position obesity management as a principal goal (ie, aiming for substantial weight loss as the primary means to treat patients with type 2 diabetes and reach glycaemic targets). Such a weight-centric intervention would disrupt the underlying pathophysiology of type 2 diabetes, reverse or slow down the disease course, concomitantly benefit other associated cardiovascular risk factors, and prevent microvascular and macrovascular complications of type 2 diabetes in the long term.
Here, we review the clinical evidence supporting weight loss as a fundamental target, propose a novel therapeutic framework, and explore challenges for the widespread implementation of this approach for people with type 2 diabetes.
Type 2 diabetes and obesity are interconnected, heterogeneous diseases
Obesity and type 2 diabetes are heterogeneous conditions. Not all people who are categorised as having obesity (ie, body-mass index ≥30 kg/m2) have excessive adiposity. Moreover, even among people who do have excess adiposity, not all people will have metabolic complications, such as type 2 diabetes.8
Conversely, some people with only minimal adiposity develop metabolic complications, prompting the concept that adipose tissue pathology, rather than quantity, might be the primary driver of complications.9
Abnormal adipose tissue pathology is characterised by adipocyte hypertrophy, visceral adiposity, and ectopic fat deposition, with resulting systemic inflammation and metabolic dysfunction. This process is not directly proportional to adipose quantity or body-mass index. For example, Asian populations tend to develop type 2 diabetes at a younger age, lower body-mass index, and following relatively little weight gain compared with White populations, most likely driven by increasing insulin resistance due to increased visceral adiposity and inadequate β-cell response.10
Although not all people with type 2 diabetes have obesity, most have abnormal adiposity, which is strongly linked mechanistically to type 2 diabetes.11
The relationship between adiposity, insulin resistance, and β-cell function is variably modulated in people of different ethnicities, further adding to the heterogeneity of type 2 diabetes.12,13
However, it is challenging to identify a large subgroup of people with type 2 diabetes who would not benefit from intentional and durable weight loss to reduce adipose tissue pathology and improve their metabolic milieu. Weight loss has benefits regardless of whether the pathology of type 2 diabetes is dominated by insulin resistance or β-cell dysfunction. Insulin resistance shares many pathophysiological pathways with obesity,11 and thus people with insulin resistance as the primary driver of type 2 diabetes will benefit most from weight loss, whereas people in whom type 2 diabetes is driven mainly by β-cell dysfunction are unlikely to have remission, but nonetheless, weight loss will minimise insulin requirements and might reduce β-cell lipotoxicity and glucotoxicity, leading to improved metabolic and glycaemic control and minimising overall treatment burden.
Several organisations 14,15 shifted from a definition of obesity that was based on weight to a definition that was based on comorbidities to account for these various phenotypes. WHO defines obesity as “abnormal or excessive fat accumulation that presents a risk to health”,15 and the American Association of Clinical Endocrinologists redefined obesity as an adiposity-based chronic disease (also known as ABCD) to emphasise its chronic nature. 14
Although these definitions might fall short by identifying only people who already have complications that are related to adiposity, their advantage lies in recognising people in whom adiposity is a driver of disease and who would benefit from a weight-loss intervention.
Our proposed therapeutic framework intends to harness weight loss as a disease-modifying intervention for people with type 2 diabetes who are identified as having an adiposity-based chronic disease that is complicated by hyperglycaemia. We term this framework to be weight-centric to acknowledge that weight loss is the most common and effective means to reverse adipose pathology. This terminology should not be misinterpreted as our advocating for a so-called normal weight target. The term adipose-centric might resonate better with some people, align with the terminology that has been proposed by American Association of Clinical Endocrinologists, and more accurately reflect the underlying pathophysiology than the term weight-centric, yet for practical reasons we propose terminology that is readily actionable as a therapeutic tool and quantifiable as a therapeutic goal by any patient or health-care provider, irrespective of available resources.
Benefits of weight loss across the disease continuum
The disease continuum for type 2 diabetes extends beyond what is captured by glycaemia. The underlying metabolic abnormalities ultimately leading to hyperglycaemia are typically present decades before a diagnosis of type 2 diabetes and are characterised by weight gain, central adiposity, and insulin resistance. The disease progresses to prediabetes, as the β cells’ ability to compensate for the increased demand that is imposed by insulin resistance diminishes, and ultimately to type 2 diabetes. As β-cell function decreases and hyperglycaemia worsens, the disease progresses to so-called end-stage type 2 diabetes (figure 2). Although the thresholds that are used to identify these clinical states do not reflect a change in underlying pathology, they are used to make treatment decisions and establish expectations for outcomes. When an intervention is started in the prediabetes stage, potential outcomes are remission of prediabetes or prevention of progression to overt diabetes, whereas if the same intervention is started after the diagnosis of type 2 diabetes, then outcomes are diabetes remission or improvement. This distinction is relevant when comparing the effectiveness of different interventions across studies, as the stage at which the intervention is started will be the key determinant of the outcome. The terminology for remission of type 2 diabetes is controversial, and there is no standard definition; therefore considerable variation exists across studies in how this endpoint is defined. 16, 17
An expert group proposed to define complete remission as normoglycaemia without glucose-lowering medication for at least 1 year, 16 but this definition is not widely used and poses implementation challenges, since many therapies to reduce bodyweight also directly lower glucose; in this context, glycaemic remission might be more appropriate.
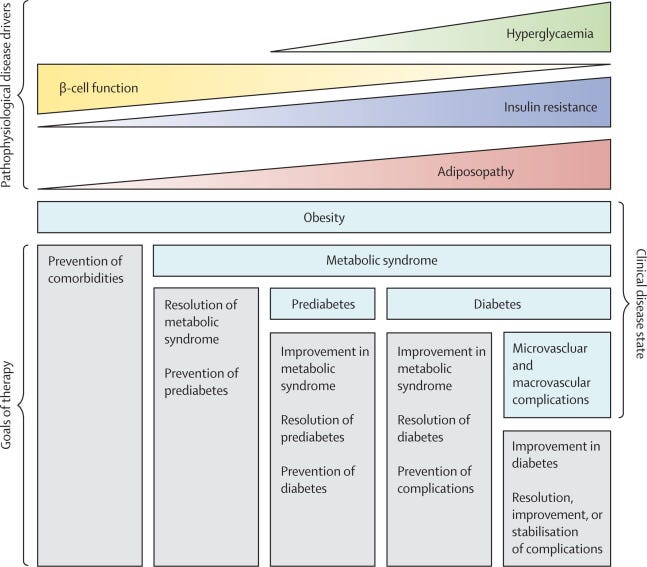
Figure 2 The disease continuum for weight-related type 2 diabetes
Nonetheless, at all disease stages, the benefits of weight loss extend beyond glycaemia to include improvements in metabolic comorbidities 18 , 19 (figure 1) and prevention or even reversal of microvascular complications that are associated with diabetes, such as chronic kidney disease. 20 , 21
Intensive lifestyle interventions for weight loss in people with type 2 diabetes
The value of weight loss in the management of patients with type 2 diabetes has long been known. 22
Studies of comprehensive lifestyle interventions have generated impressive data regarding glycaemic control and even remission of type 2 diabetes. The DiRECT randomised controlled trial (RCT) evaluated an intensive dietary intervention in 306 adults with body-mass index of 27–45 kg/m2 and type 2 diabetes with a duration less than 6 years. 7
After 2 years of follow-up, 11% (17 of 149) of people on the dietary intervention lost at least 15 kg, compared with 2% (three of 149) of people in the routine-care control group. In a post-hoc analysis of 272 participants for whom 24-month data were available, 70% (14 of 20) of people who lost at least 15 kg had diabetes remission (ie, defined as glycated haemoglobin [HbA1c] of <6·5% [<48 mmol/mol] after at least 2 months off glucose-lowering medications). 23
By contrast, 60% (15 of 25) of people who lost between 10 kg and less than 15 kg, 29% (21 of 73) of people who lost between 5 kg and less than 10 kg, and 5% (eight of 154) of participants who lost less than 5 kg (ie, from baseline weight of approximately 100 kg) at 2 years had diabetes remission. People in the intensive intervention group who did not have remission from type 2 diabetes still had a greater HbA1c reduction, and required fewer glucose-lowering agents than did the control group. 23
Although this study is limited by enrolment of only 20% (306 of 1510) of screened candidates and attrition of nearly a third (48 of 150) of intervention participants during follow-up, its relevance stands in establishing the strong correlation between magnitude of weight loss and likelihood of remission from type 2 diabetes and showing that loss of 15% of bodyweight can result in remission in most patients with early type 2 diabetes.
The Look AHEAD RCT randomly assigned 5145 adults with type 2 diabetes to intensive lifestyle modification aimed at 7% weight loss or to usual care. After 4 years of follow-up, participants in the intensive lifestyle group had lost a mean of 4·7% of bodyweight versus 0·8% in the control group, 24 but few (ie, 7% in the intervention group vs 2% in the control group) people had an HbA1c of less than 6·5% (<48 mmol/mol), suggesting that greater weight loss is required for a meaningful effect on the disease course of type 2 diabetes. Interestingly, although at the end of the study (ie, approximately 10 years of follow-up; mean weight loss of 6% in the intervention group vs 3·5% in the control group) the primary endpoint of cardiovascular events was not significantly different between groups, 25 a post-hoc analysis showed that the 21% (1013 of 4899) of participants who lost at least 10% of their bodyweight in the first year had a 21% lower risk of cardiovascular events over 10 years than did people with stable weight or weight gain.26
Such studies support the benefits of losing more than 10% of bodyweight on the disease process of type 2 diabetes, diabetes-related endpoints, and complications in the long term, including cardiovascular events. They also emphasise that mean weight losses in the long term with lifestyle interventions often fall short of what clinicians and patients might hope for, and few participants, usually two in every ten participants, respond to lifestyle interventions in the long term, even with intensive support. 23
As such, eight in ten people will require additional interventions for significant weight loss and maintenance. These numbers are not surprising, because obesity is not simply the result of a poor lifestyle.
Challenges of maintaining weight loss in the long term
The emerging pathophysiology of obesity as a chronic disease with dysregulation of appetite at the level of the brain’s subcortical areas helps to explain the counter-regulatory mechanisms that promote weight regain in response to calorie reduction. Weight loss that is induced by dieting causes a multitude of physiological changes that seem to impede the sustained reduction in energy intake that is required for weight loss in the long term. 27 , 28
The resulting increase in drive to eat and reduction in energy expenditure create ideal conditions for weight regain, particularly within an environment that promotes obesity (eg, ready access to low-quality, high-calorie, fast food and decreased physical activity).
Most lifestyle interventions result in progressive weight loss over 6 months, followed by plateau and weight regain over 1–3 years, 29 although continued monitoring, lifestyle counselling, and anti-obesity medications can prolong maintenance of weight loss. 30
Medications to reduce appetite partly counteract the increased drive to eat and impaired satiation associated with weight loss that is induced by dieting but, as with most management techniques for chronic diseases, are only effective while in use. 31 , 32
Interindividual variability in response is another major challenge, because there are no reliable pretreatment predictors of weight loss or its subsequent effect on type 2 diabetes. However, it has been consistently observed that all obesity treatments lead to less weight loss in people with type 2 diabetes than in people without diabetes. The underlying reasons are unclear, although potential explanations include decrease in glycosuria (and thus calorie loss) with improved glycaemic control, use of concomitant medications that promote weight gain (eg, sulfonylureas, insulin, and beta blockers), and a genetic background that predisposes to weight gain. 33
Successful strategies to facilitate substantial weight loss in the long term will need to disrupt the biological mechanisms that drive obesity.
Bariatric surgery
Bariatric surgery is an established, effective treatment for obesity in people with type 2 diabetes. It decreases blood glucose and allows decreased use of glucose-lowering medications within days of surgery, effectively placing type 2 diabetes into remission in up to 75% of patients in the short term to midterm (ie, up to 5 years) 34 and in 37–51% of patients over the long term (ie, up to 20 years). 35 , 36
Several randomised trials (table 1) comparing bariatric surgery with best medical care have shown that bariatric surgery leads to higher rates of type 2 diabetes remission and improves glycaemic control in the long term. Furthermore, bariatric surgery also leads to improvements in other metabolic comorbidities that are related to obesity, such as abnormal blood pressure and concentrations of triglyceride, LDL, and HDL cholesterol. Mean weight loss ranged from 22% to 37% after Roux-en-Y gastric bypass and was 19% after sleeve gastrectomy, compared with 1–10% in medical treatment groups.
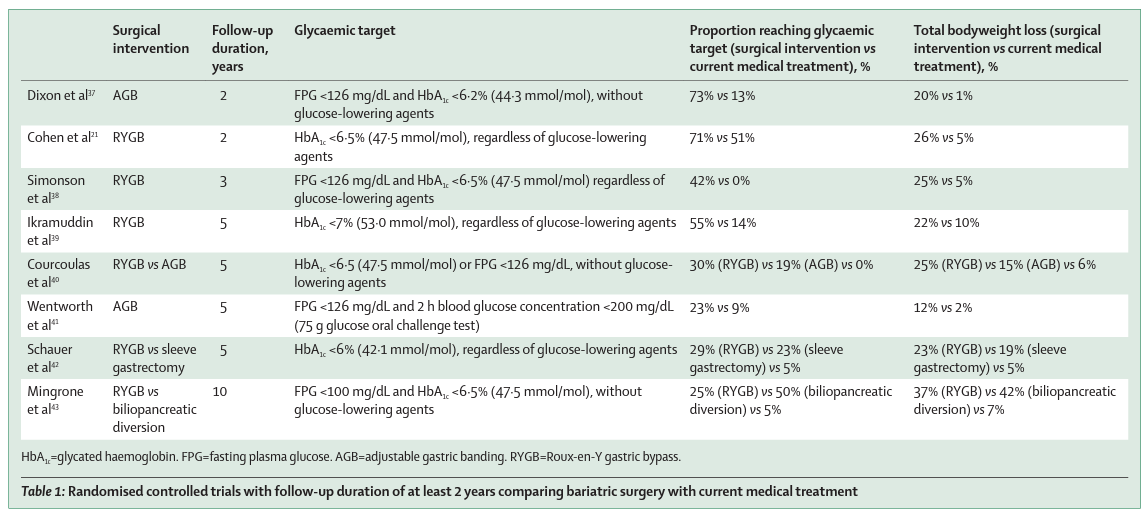
A 12-year follow-up of a prospective observational study 36 showed that Roux-en-Y gastric bypass resulted in sustained 27% total weight loss and a lower incidence of type 2 diabetes (eight [3%] of 303) compared with a non-surgical treatment group (2% total weight loss; 89 [26%] of 348 had type 2 diabetes). Among people with type 2 diabetes at baseline, 51% (43 of 84) were still in remission at 12 years. Observational studies over a long period of time and RCTs also show downstream benefits of bariatric surgery on type 2 diabetes far beyond glycaemic control, including remission of early stage chronic kidney disease 21 and reduction in microvascular events by 56% and macrovascular events by 32% over 20 years of follow-up. 35
Several matched cohort studies also noted a reduction in major adverse cardiovascular events in patients with type 2 diabetes after bariatric surgery. 44 , 45 , 46 , 47
In the RCT with the longest follow-up to date, Mingrone and colleagues compared outcomes of Roux-en-Y gastric bypass and biliopancreatic diversion with medical treatment in people with type 2 diabetes. 43
At 10 years, the surgical intervention groups compared with the medical intervention group had sustained double-digit weight loss, a greater than ten-times lower risk of microvascular complications, and improved quality of life. 43
Weight loss is the most significant predictor for remission of type 2 diabetes, along with age, diabetes duration, and insulin use. 48
In the Swedish Obese Subjects Study, remission rates for type 2 diabetes after 2 years in the entire cohort (ie, surgical and control groups) were 97% (210 of 217) in people with a body-mass index reduction of more than 15 kg/m2, 78% (589 of 755) in people with 10–14 kg/m2 loss, 60% (590 of 983) in people with 1–9 kg/m2 loss, 20% (249 of 1247) in people with no weight change (ie, <1 kg/m2), and 11% (31 of 283) in people who gained weight. 49
Although weight loss by any means strongly predicts metabolic improvement, bariatric surgery also exerts effects that are independent of weight loss and induce alterations in the release of hormones to regulate appetite from the gastrointestinal tract, which affect feeding behaviour via the gut–brain axis and can also have direct glucose-lowering effects. 50
Bariatric surgery is included in the treatment algorithms of the International Diabetes Federation and the American Diabetes Association, 51, 52 yet it is not without risks (figure 3). Furthermore, it is not feasible for a condition as prevalent as type 2 diabetes to be routinely managed with invasive surgery. Nevertheless, a key learning from bariatric surgery is that, if obesity is effectively treated and weight loss of more than 15% is reached in the long term, then metabolic control can be sustained and metabolic complications, including microvascular and macrovascular complications, can be prevented. 35 , 46 , 56
Until now, there were no non-invasive tools to replicate such effects, but this situation is rapidly evolving.
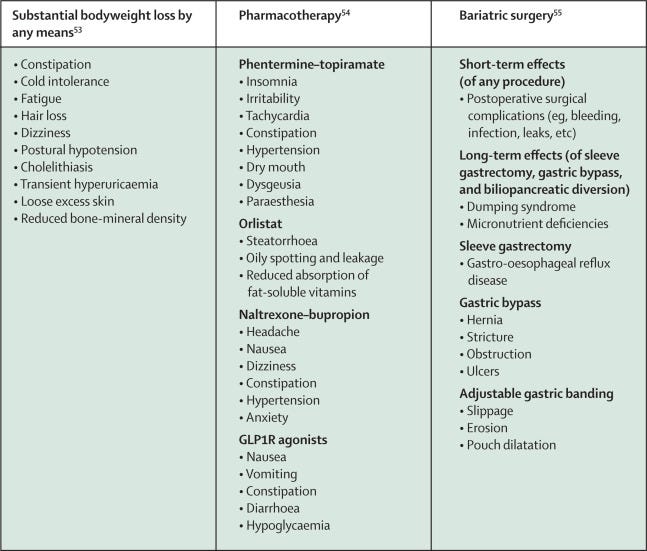
Figure 3 Most common adverse effects of substantial weight loss by any means, pharmacotherapy, and bariatric surgery
Pharmacotherapy associated with weight loss in type 2 diabetes
Five agents (ie, orlistat, phentermine–topiramate, naltrexone–bupropion, liraglutide 3·0 mg, and semaglutide 2·4 mg) are approved by one or more regulatory authorities worldwide for chronic weight management. Additionally, phentermine is approved for use in the short term (ie, up to 3 months), but its risk–benefit profile for chronic use is not favourable, although as with most anti-obesity medications, some people lose substantial weight. 57
Phentermine is widely prescribed, mainly in the USA, but in spite of its widespread use, the longest published placebo-controlled trial of phentermine lasted 36 weeks. 58
Orlistat has been approved for obesity treatment for more than 20 years. Its modest weight-reducing effect results from reducing absorption of ingested fat by approximately 30%. 59
Over the past decade, several agents that act centrally to reduce hunger or promote satiation have become available, including combinations of bupropion plus naltrexone, 60 phentermine plus topiramate, 61 and the GLP1R agonist liraglutide. 62
There are no published direct comparisons of these classes, but clinical trials indicate that, on average, these newer agents facilitate placebo-subtracted weight losses of 3–7% at 12 months in people with type 2 diabetes (table 2). A 2016 systematic review and meta-analysis, including studies in people with and without type 2 diabetes, showed that of these agents, phentermine–topiramate was associated with the highest odds of losing at least 10% bodyweight (54% of participants). 65
A direct correlation was noted between effectiveness for lowering weight and the probability of adverse events. The nature of adverse effects varies across agents (figure 3), but overall discontinuation rates were lower in intervention groups than in the placebo group for all classes. 65
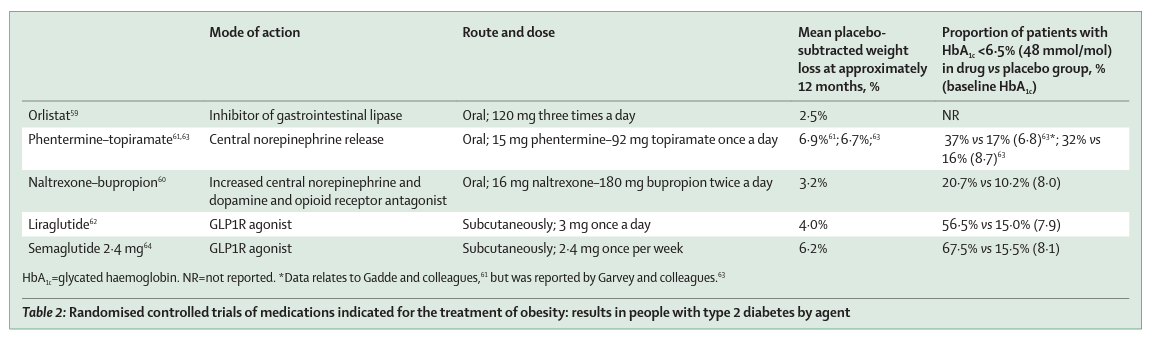
2·4 mg semaglutide per week was approved by the US Food and Drug Administration for chronic weight management in June, 2021, on the basis of the STEP clinical trials results. In participants with type 2 diabetes (n=1210; STEP 2), 64 68 weeks of subcutaneous semaglutide 2·4 mg weekly was associated with placebo-subtracted weight loss of 6·2%, HbA1c reduction of 1·6% (17·5 mmol/mol; vs 0·4% [4·1 mmol/mol] in the placebo group), and reaching HbA1c less than or equal to 6·5% (≤48 mmol/mol) in 68% (257 of 381) of participants from a baseline level of 8·1% (65 mmol/mol). Notably, mean weight loss appeared lower than in people without type 2 diabetes (STEP 1, placebo-subtracted weight loss 12·4% at week 68), 66 yet more than a quarter (100 [26%] of 388) of participants with type 2 diabetes had a mean weight loss of at least 15%, compared with 3% (12 of 376) in the placebo group.64
Besides these obesity pharmacotherapies, four of the 12 classes of glucose-lowering agents that are approved for type 2 diabetes are recognised to promote weight loss: SGLT2 inhibitors, GLP1R agonists, biguanides (metformin), and amylin analogues (pramlintide). The average weight loss that is observed with these drug classes is modest (ie, 1·4–1·9 kg, with HbA1c reductions of 0·4–0·9%) in adults with type 2 diabetes over 6–12 months of treatment, 67, 68 , 69 although there is considerable interindividual variability in response. There are few direct comparisons between available agents; however, semaglutide at a subcutaneous dose of 1 mg per week appears to be associated with greater reductions in weight and HbA1c than are other relevant active comparators (including exenatide 2 mg, canagliflozin 300 mg, and liraglutide 1·2 mg), with a placebo-subtracted weight loss of approximately 5% (ie, similar to most medications that are approved for a weight loss indication, aside from semaglutide 2·4 mg) and mean reductions in HbA1c of 1·4% (15 mmol/mol). 70
Furthermore, in a premarketing cardiovascular outcome study in patients with type 2 diabetes and cardiovascular disease or high cardiovascular risk, semaglutide 1·0 mg (compared with placebo) was associated with a 26% reduction in the primary endpoint of major adverse cardiac events. 71 A systematic review and network meta-analysis of RCTs 72 reported that semaglutide is the most effective agent to reduce bodyweight (ie, 11·41% mean reduction in bodyweight across all doses), followed by phentermine–topiramate (ie, 7·97% mean reduction in bodyweight across all doses), both agents having overall similar risk of adverse events compared with the other weight-reducing agents.
Weight loss of approximately 5%, as attainable with many of the available anti-obesity and some glucose-lowering agents, brings about improvements in complications that are related to adiposity, and the available agents are all associated with amelioration of the cardiometabolic risk profile. 73
Nonetheless, with the available pharmacotherapies, only a minority of people have and sustain the substantial weight loss that is required to materially alter the course of type 2 diabetes, even with concurrent intensive lifestyle interventions, which are particularly challenging to maintain outside a clinical trial setting.
Pharmacotherapy pipeline for obesity and potential role in type 2 diabetes
Several agents in development that replicate the action of gut-derived satiety hormones have the potential to change the current landscape, making sustained, substantial weight loss a realistic consideration as a primary goal in treatment for people with type 2 diabetes (appendix p 1). They are particularly appealing in type 2 diabetes because of their additional glucose-lowering effect that is independent of weight.
Dual and triple agonists
Unimolecule or multimolecule combinations with dual and triple agonist action, combining GLP1 with GIP, glucagon, or both, aim to exploit the complementary biological actions of gut peptides on food intake and mimic the endogenous coordinated release of several gut hormones postprandially (ie, an effect that is harnessed for therapeutic benefit by some bariatric procedures).
Tirzepatide, a novel dual GLP1 and GIP analogue, is being investigated at weekly subcutaneous doses of 5 mg, 10 mg, and 15 mg for patients with type 2 diabetes in the SURPASS phase 3 clinical trial programme. All three doses of tirzepatide resulted in larger reductions in bodyweight (ie, 5 mg dose led to 7·6 kg [8·2%] loss, 10 mg dose led to 9·3 kg [9·3%] loss, and 15 mg dose led to 11·2 kg [11·9%] loss) and HbA1c (ie, 5 mg dose led to 2·0% reduction, 10 mg dose led to 2·2% reduction, and 15 mg dose led to 2·3% reduction) than did semaglutide 1·0 mg (ie, 5·7 kg [6·1%] bodyweight loss and 1·9% HbA1c reduction) over 40 weeks as an add-on to metformin (appendix p 1). 74
Most participants had HbA1c lower than 6·5% (<48 mmol/mol; ie, 69–80% of participants in the tirzepatide group vs 64% in the semaglutide groups), indicating that previously aspirational treatment targets are becoming a reality, even in people with long-standing type 2 diabetes (baseline mean HbA1c 8·3% [67 mmol/mol]; type 2 diabetes duration 8·6 years). Equally impressive results were noted in all reported trials to date. 75 , 76 The combination of GLP1R and glucagon receptor (GCGR) agonists aims to capitalise on the effect of both glucagon and GLP1 on satiety, while counterbalancing glucagon’s mobilisation of hepatic glucose with GLP1’s stimulation of insulin secretion. Results so far have been disappointing. A phase 2b study of cotadutide, a synthetic peptide with balanced dual GLP1R and GCGR agonist activity, 77 in people with overweight or obesity and type 2 diabetes who were on metformin monotherapy showed mean reductions in bodyweight of 5·0% with the highest dose of cotadutide 300 mg (vs 3·3% with liraglutide 1·8 mg and 0·7% placebo), reduction in HbA1c of 1·2% (vs 1·2% and 0·5%), and 39% of participants (vs 38% and 11%) reaching HbA1c lower than 6·5% (<48 mmol/mol) at 54 weeks. Another GLP1R and GCGR dual agonist (BI456906) has started in phase 2 clinical trials for the treatment of people with type 2 diabetes, obesity, and non-alcoholic steatohepatitis (NCT04153929, NCT04667377, NCT04771273).
Amylin agonists
In contrast to pramlintide, which is short acting and requires multiple daily injections, cagrilintide is a weekly subcutaneous amylin analogue that is under development for the treatment of obesity. In a 26-week phase 2 study of 706 patients without type 2 diabetes who were randomly assigned to cagrilintide 0·3–4·5 mg (five groups receiving different doses; n=100–102 per group), liraglutide 3·0 mg (n=99), or placebo (n=101), cagrilintide led to progressive, dose-dependent weight reductions that had not plateaued by week 26 and greater weight loss at all doses than with placebo (appendix p 1). 78
Moreover, weight loss was greater with cagrilintide at the 4·5 mg dose than with liraglutide 3·0 mg (ie, 10·8% vs 9·0%). 53·5% of participants lost more than 10% bodyweight and 18·7% of participants lost more than 15% bodyweight with cagrilintide 4·5 mg. Gastrointestinal disorders were the most common adverse events. Cagrilintide was also evaluated in a dose-finding phase 1b study in combination with semaglutide 2·4 mg. Cagrilintide 2·4 mg and semaglutide 2·4 mg led to 17·1% bodyweight loss compared with 9·8% loss with semaglutide 2·4 mg plus placebo at 20 weeks, after only 4 weeks at the maximum dose (appendix p 1). 79
This increased loss was not accompanied by worsening tolerability, suggesting that the two complementary mechanisms of action might be combined in a fixed-dose product for potential additive weight loss that could reach the range of 20–25% bodyweight loss.
A novel therapeutic framework: sustained weight loss as a primary treatment goal in type 2 diabetes
With the promise of new options, it is time to consider shifting the treatment focus for patients with type 2 diabetes from the current reactive glucocentric approach to addressing obesity, the core driver of insulin resistance and contributor to β-cell failure.
The evidence that sustained double-digit weight loss can reverse the pathophysiological underpinnings of type 2 diabetes is at a similar level of maturity as was the evidence for prevention of cardiovascular events when the previous shift in treatment goals occurred. We contend that attaining and maintaining substantial weight loss (ideally >15%) should be repositioned as the initial principal treatment goal for a sizeable subset of people with type 2 diabetes. The clinical benefits of weight loss are on a continuum (appendix p 2), but weight loss of at least 15% has a greater likelihood of disrupting the disease course of type 2 diabetes than does a smaller loss. This concept is supported by the DiRECT study, in which optimal type 2 diabetes remission was reached in participants with at least 15% weight loss.23
Moreover, the mortality benefit of bariatric surgery is evident with more than 15% weight loss, irrespective of the technique through which it is reached. 80
Studies of novel pharmaceuticals, such as semaglutide 2·4 mg and tirzepatide 15·0 mg, have reported that 15% of bodyweight can be readily lost in more than 25% of patients with type 2 diabetes, and that such weight loss is associated with near normalisation of glycaemic control in most participants. 64 , 74 , 75 , 76
We believe there are likely to be benefits of reducing adipose tissue in people with type 2 diabetes irrespective of the starting quantity. This concept is similar to the rationale for lowering LDL cholesterol in patients with type 2 diabetes, where the advantages are evident irrespective of starting LDL concentration. The benefits of a weight-centric approach to type 2 diabetes will be greatest in people whose type 2 diabetes is primarily driven by insulin resistance; hence this approach should be prioritised in this subpopulation. However, weight loss promotes glycaemic control and improvement in cardiovascular risk factors in most patients with type 2 diabetes and, therefore, should continue to be a key part of the multifaceted approach to type 2 diabetes and cardiovascular risk management in most patients. Approaches to induce substantial weight loss will need to be monitored to ensure that the benefits outweigh the risks (figure 3).
We propose that the primary treatment goal should be determined by the predominant type 2 diabetes phenotype (appendix p 3, table 3). We acknowledge that current tools do not allow easy detection of adipose tissue pathology in clinical practice, and that body-mass index is a suboptimal predictor of which patients would benefit from substantial weight loss. This challenge is akin to the one faced when trying to differentiate between type 1 and type 2 diabetes. Although most patients will have a clear phenotype, in some patients differentiation is not straightforward or even possible. Until improved tools become available, we recommend that, for the subset of patients who appear to have a primarily β-cell defect-driven disease, it is appropriate to focus primarily on lowering blood glucose concentration. In people with type 2 diabetes and established atherosclerotic cardiovascular disease, the primary goal is reducing the risk of further major adverse cardiovascular events, and as such — in line with the current treatment guidelines that were proposed by the American Diabetes Association, the European Association for the Study of Diabetes, and cardiology societies 81 , 82 , 83 — the use of SGLT2 inhibitors or GLP1R agonists with known cardiovascular benefits should be prioritised. A similar approach should be followed in patients who have heart failure with reduced ejection fraction or chronic kidney disease, where SGLT2 inhibitors with proven benefits should be prioritised. 84
Most others with type 2 diabetes will have one or more features of insulin resistance, consistent with the adiposity-associated diabetes phenotype (regardless of body-mass index), and are likely to benefit from a weight-centric treatment approach. Salient features that identify people in whom adiposopathy is a key mechanistic contributor to type 2 diabetes are the presence of central adiposity, increased waist circumference, acanthosis nigricans, multiple skin tags, hypertension, hypertriglyceridaemia, non-alcoholic fatty liver disease, or — if available — laboratory evidence of hyperinsulinaemia. In this population, we propose a treatment goal of total weight loss of at least 15%, with the intention of not merely improving glycaemia, but rather as the most effective way to disrupt the core pathophysiology of type 2 diabetes and thus change its course in the long term and prevent its associated metabolic complications. As such, in this subpopulation, interventions with the greatest potential to facilitate sustained weight loss should be prioritised, followed by pharmacotherapy that reduces or prevents cardiovascular complications of type 2 diabetes and additional agents that facilitate glycaemic control. If additional pharmacotherapy is required to reach glycaemic goals, then weight-neutral agents (ie, agents that do not promote weight gain) should be favoured.
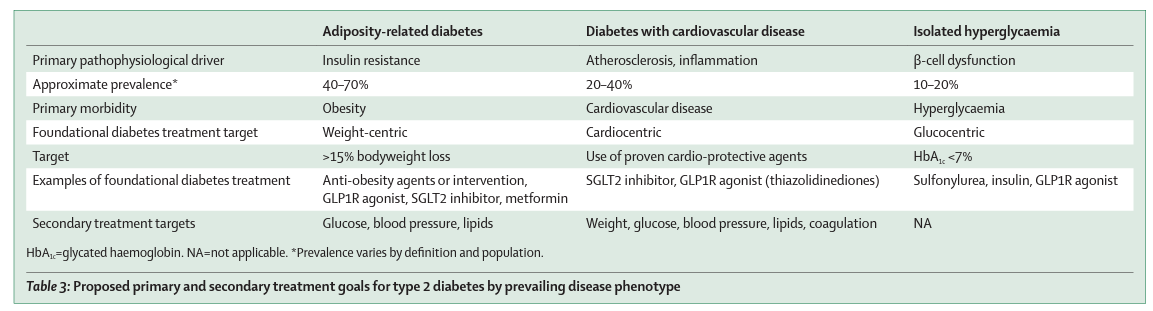
Young people or people who are newly diagnosed with type 2 diabetes are more likely to be categorised in this subgroup of patients in whom adiposopathy is a key mechanistic feature, yet the benefits of this weight-centric approach extend irrespective of diabetes duration and should therefore be considered even in people with long-standing type 2 diabetes.
We acknowledge that no randomised studies show benefits of weight loss compared with standard care on cardiovascular outcomes in patients with type 2 diabetes. However, evidence supporting reduction in macrovascular and microvascular complications with weight loss is mounting. Several RCTs have reported on these endpoints in secondary analyses, 85 as have high-quality prospective observational studies, 36 such as the EPIC-Potsdam study, 86 which observed that weight loss was associated with reduction of microvascular complications in patients with type 2 diabetes irrespective of baseline body-mass index.
In the same manner that addition of cardiovascular risk reduction as a central goal for patients with type 2 diabetes and established cardiovascular or renal disease 87 , 88 did not replace the need to optimise glycaemic control, it is important to stress that a new weight-centric goal would not exclude the parallel goal of glycaemic control nor replace other evidence-based strategies for decreasing the risk of microvascular and macrovascular complications of type 2 diabetes. Although in most cases treating obesity for double-digit weight loss will improve glycaemia, further glucose-lowering strategies should be added to maximally reduce microvascular risk, either sequentially if glycaemic targets are not met by weight loss or concurrently if there is clinical urgency. In all patients, even those who reach glycaemic targets or diabetes remission, the risk of microvascular and macrovascular complications should continue to be managed comprehensively according to existing evidence. Thus, management of obesity is not a competitor but an addition to existing treatment goals. However, the shift in emphasis to prioritise weight loss is important as a proactive approach to addressing a key driver of the disease process of type 2 diabetes that will have benefits well beyond lowering glucose alone.
Practical considerations for making sustained weight loss a primary treatment goal
There are important considerations when redefining treatment goals for patients with type 2 diabetes to focus on sustained weight loss. Firstly, the initiative should be driven by updating treatment guidelines to include not only the emerging evidence for remission of type 2 diabetes after double-digit weight loss by lifestyle intervention, pharmacotherapy, and bariatric surgery but also the specific focus on substantial, sustained weight loss as a primary treatment target for patients with type 2 diabetes, replacing the exclusive focus on glycaemic control that has been long held.
Secondly, public and private payers should invest in their constituency’s health to derive savings in the long term from minimising costly downstream metabolic complications. Health economic models that are used to calculate utility gains during health economic assessments or health technology appraisals should be adjusted to allocate values to 10%, 15%, and 20% weight loss as these goals now become more widely accessible for the first time. The broad health benefits in the long term of substantial weight loss should offset the needed upfront investment in effective weight-loss interventions.
Thirdly, regulators and payers should avoid promoting an arbitrary dichotomy between approval pathways, reimbursement for medications, and care provision for people with obesity and people with type 2 diabetes. These two conditions are intricately related, and it is important to recognise that the upstream intervention of weight loss is the most effective treatment for patients with type 2 diabetes. As such, established and emerging anti-obesity agents have a crucial role in the management of patients with type 2 diabetes, particularly patients with weight-independent benefits on glycaemia. It is also well recognised that chronic diseases, such as type 2 diabetes, hypertension, and hyperlipidaemia, are unlikely to be successfully treated with a single medication. Combinations of agents with complementary mechanisms of action are generally more effective and have fewer dose-limiting adverse effects than do maximum monotherapies. This concept should be applied in obesity management as well and has already been shown with several combination products available and more being developed.
Fourthly, equitable access should be ensured to effective interventions so that health-care disparities are not exacerbated. Type 2 diabetes, obesity, and their debilitating complications affect people across all socioeconomic circumstances and regions. Although there are ethnic and racial variations in the relationship between adiposity and its metabolic consequences, all subgroups with type 2 diabetes and obesity are likely to benefit from effective treatments. Therefore, ensuring access to these options is not limited to people in high-income settings is of paramount importance.
Finally, practice management should refocus to effectively incorporate weight management to treat patients with type 2 diabetes. Health-care providers, especially those managing diabetes routinely, should be trained and become experienced in all aspects of obesity management. Support staff should be trained to support patients through their weight-loss journeys, and practices should consider the need for specialised staff to deliver the educational component of the new treatment strategies that are proposed. Since scientific knowledge about obesity and bodyweight regulation is new, having advanced rapidly since the discovery of leptin in 1994, many health-care providers might not be familiar with the science of obesity or even with approaching obesity as a chronic disease. People with type 2 diabetes and obesity are accustomed to the widely held perception that these diseases are self-induced and could be cured simply by eating less and doing more physical activity. Therefore, they might be reluctant to ask health-care professionals for help due to misconceptions that the responsibility for weight loss is entirely their own or that they will be blamed for having difficulty. Furthermore, until 2021, losing and maintaining the loss of 15% or more bodyweight was not considered realistic without bariatric surgery; therefore, many health-care providers and patients might still regard obesity treatment from a viewpoint of therapeutic nihilism, which will need to be updated as treatment options evolve to make substantial weight loss an attainable goal.
We acknowledge that many people with type 2 diabetes might not want to use dietary interventions, medications, or surgery for treatment of obesity. Moreover, even if these interventions were highly sought after, some, such as surgery or even life-long injectable medications, would be difficult to scale to reach everyone who could potentially benefit. One of the major contributions of bariatric surgery is showing that strategies that result in substantial and durable weight loss can disrupt the disease course of type 2 diabetes, adding to the drive to develop more effective non-invasive interventions. As these treatments become available, patients will have more choices. These alternatives will be presented to them only if the clinical community embraces the evidence-based idea of substantial weight loss as an effective treatment for patients with type 2 diabetes.
Conclusions
The time is right to consider the addition of substantial (ie, double-digit) weight loss as a principal target for the treatment of many patients with type 2 diabetes. This approach would address the pathophysiology of the disease process for type 2 diabetes; recognise adipose tissue pathology as a key underlying driver of the continuum of obesity, type 2 diabetes, and cardiovascular disease; and reap metabolic benefits far beyond glycaemia. Such a change in treatment goals would recognise obesity as a disease with reversible complications and require a shift in clinical care.
Search strategy and selection criteria
See long version.
Contributors
All authors contributed equally to conceptualisation, literature search, manuscript preparation, figures, and tables.
Declaration of interests
IL received research grants (paid to the institution) from Novo Nordisk, Sanofi, Boehringer Ingelheim, Merck, Pfizer, Mylan, and the National Institutes of Health; participated in scientific advisory roles or engaged in consulting for Novo Nordisk, Eli Lilly, Sanofi, AstraZeneca, Boehringer Ingelheim, Janssen, Intercept, Intarcia, TARGETPharma, Mannkind, Valeritas, Merck, Bayer, and Zealand Pharma; received non-financial support from Novo Nordisk, Eli Lilly, Sanofi, AstraZeneca, Boehringer Ingelheim, Janssen, TARGETPharma, Merck, and Pfizer. PS received a research grant (paid to the institution) from the National Health and Medical Research Council; received honoraria for lectures; and participated in scientific advisory boards for Novo Nordisk and is an Australian and New Zealand Obesity Society council member (unpaid role). RVC received research grants from Johnson & Johnson Medical Devices Brazil; received honoraria for lectures from Johnson & Johnson Brazil, Medtronic, Janssen Pharmaceutical; and participated in scientific advisory boards for Johnson & Johnson Brazil, Medtronic, GI Dynamics, Novo Nordisk, Abbott, Baritek, and Keyron. CWlR received research grants (paid to the institution) from the Irish Research Council and Health Research Board; received consulting fees and honoraria for lectures; participated in scientific advisory boards; received non-financial support from Novo Nordisk, Herbalife, and Johnson & Johnson; and holds a leadership role (unpaid) in the Irish Society for Nutrition and Metabolism.
Acknowledgments
We thank Ludmilla Dellatorre Teixeira for expert editorial help.
Supplementary Material
See long version.
Authors affiliations
Division of Endocrinology, Department of Internal Medicine and Department of Population and Data Sciences, University of Texas Southwestern Medical Center, Dallas, TX, USA
(Prof I Lingvay MD);
Department of Medicine (St Vincent’s Hospital), University of Melbourne, Melbourne, VIC, Australia
(P Sumithran PhD);
Department of Endocrinology, Austin Health, Melbourne, VIC, Australia
(P Sumithran);
The Center for Obesity and Diabetes, Oswaldo Cruz German Hospital, São Paulo, Brazil
(R V Cohen MD);
Diabetes Complications Research Centre, Conway Institute, School of Medicine, University College Dublin, Dublin, Ireland
(Prof C W le Roux MD);
Diabetes Research Centre, Ulster University, Coleraine, UK
(Prof C W le Roux)
References
See long version.
Originally published at
PDF version
chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/viewer.html?pdfurl=https%3A%2F%2Fwww.thelancet.com%2Faction%2FshowPdf%3Fpii%3DS0140-6736%252821%252901919-X&clen=1157311












