This is a republication of the article “Optimizing data to improve the fight against HIV”, with the title above.
health transformation institute (HTI)
research institute & knowledge portal
Joaquim Cardoso MSc*
Founder, and Chief Researcher & Editor
December 1, 2022
MSc* from London Business School
MIT Sloan Masters Program
Senior Advisor for Health Transformation & Digital Health
The importance of data in the evolution of contemporary HIV care and treatment

Oracle Health
Virginia Burke, James Willig
December 1, 2022
Modern antiretroviral therapy has a significant impact on viral replication, to the point that the treatment goal now is to achieve a HIV viral load (VL) that’s “undetectable.”
When a VL is undetectable, viral replication is suppressed and there are fewer than 20 HIV virus copies/mL in an individual’s blood.
Evidence indicates that undetectable equals untransmissible, which means individuals who achieve a suppressed or undetectable VL cannot transmit the infection to others. 1
This is incredible progress.
Theoretically, if we could quickly diagnose all individuals living with HIV, provide them with appropriate treatment, and decrease their VL to undetectable levels, we can help prevent new HIV transmissions.
Given these great strides, one might ask: Why have we still not stopped HIV/AIDS transmission?

Persistent barriers to care
The answer to the above question is complicated.
The HIV care continuum is one framework that illustrates continued barriers to limiting the spread of HIV/AIDS in our communities.

Figure 1 2 indicates that in the US in 2019, 87% of the estimated number of people living with HIV (PLHIV) had been diagnosed, yet only 57% had achieved viral suppression.
This means that in 2019, approximately 43% of PLHIV in the US could still transmit the virus.
…in the US in 2019, 87% of the estimated number of people living with HIV (PLHIV) had been diagnosed, yet only 57% had achieved viral suppression.
This means that in 2019, approximately 43% of PLHIV in the US could still transmit the virus.
Figure 1: Prevalence-based HIV care continuum in the US and six dependent areas (2019)
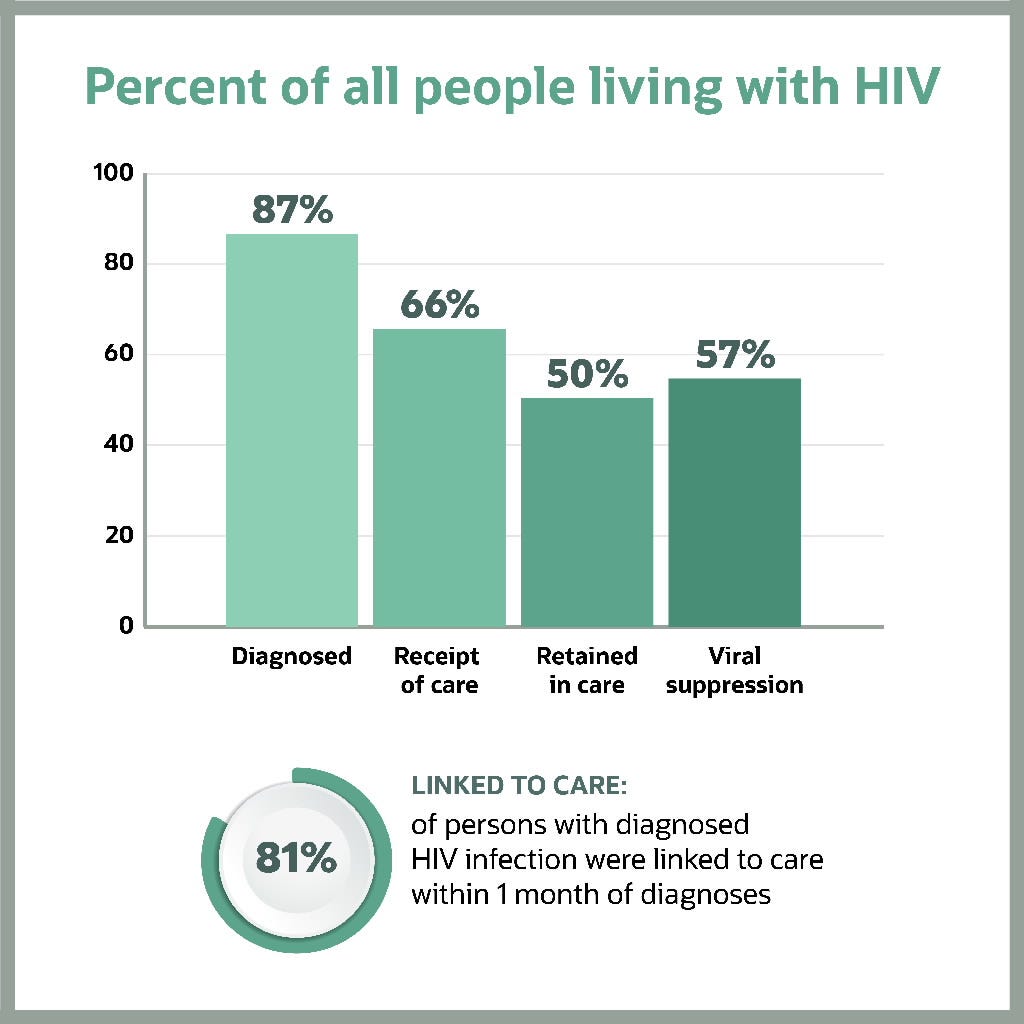
Note: Receipt of medical care was defined as ≥2 tests (CD4 or VL) ≥3 months apart in 2019. Viral suppression was defined as <200 copies/mL on the most recent test in 2019. Linkage to care is defined as having ≥one CD4 or VL test within 30 days (1 month) of diagnosis. (Linkage is calculated differently from the other steps in the continuum, and cannot be directly compared to other steps.)
Experts have dedicated their entire careers to understanding and addressing barriers to care at each stage of the HIV care continuum, striving to shift patients from the columns on the left (Diagnosis) to those on the right (Viral suppression) of the continuum.
These barriers are complex and may vary based on geography, race and ethnicity, gender, insurance status, and language, among many other factors, some of which are included in the table below 3.
These barriers are complex and may vary based on geography, race and ethnicity, gender, insurance status, and language, among many other factors, some of which are included in the table below
HIV care continuum metrics clearly demonstrate there’s still significant work ahead while providing insight into the nature of the remaining challenges.
We cannot successfully end the epidemic simply by writing more prescriptions in traditional healthcare settings; rather, we must understand the various barriers experienced by individuals and health systems across the HIV care continuum to effectively identify and implement solutions. To do this, we need high-quality data.
We cannot successfully end the epidemic simply by writing more prescriptions in traditional healthcare settings; …
… rather, we must understand the various barriers experienced by individuals and health systems across the HIV care continuum to effectively identify and implement solutions.
To do this, we need high-quality data.
Table 1: HIV/AIDS continuum of care barriers in rural communities in the US and Canada
Barriers across the continuum of care in nonurban settings
- Shortage of healthcare providers and limited HIV expertise
- Scarcity of funding for widespread testing and treatment programs in nonurban areas of the US and Canada
- Lack of systematic harm reduction programs to address the root causes of HIV acquisition (e.g., opioid addiction, trauma, and poverty)
- Increased geographic distance to providers and lack of transportation options
- Insufficient HIV awareness and knowledge at the community level
- High rates of poverty, low educational attainment, substandard housing, and food insecurity
- Stigma, isolation, and heightened fear of discrimination
- Socially conservative climate, racism, and anti-immigrant sentiment
- Limited community leadership and political support

Cross-setting data challenges and opportunities
- 1.Point of care
- 2.Research recruitment
- 3.Cross-clinic collaboration
- 4.Public health systems
1.Point of care
HIV/AIDS is unique compared to other chronic medical conditions because the virus can become resistant to therapy.
Thus, a provider would optimally know both a person’s HIV treatment history and obtain copies of any prior resistance panels before starting a new treatment regimen.
Unfortunately, to obtain information from previous care sites, many providers must rely on faxes or PDFs scanned into the patient’s electronic health record (EHR) for relevant and necessary patient information.
This unstructured but critical data is often embedded within 100-page documents, and providers are expected to identify key information and develop appropriate treatment plans within the 30-minute appointment slot scheduled for each patient.
Unfortunately, to obtain information from previous care sites, many providers must rely on faxes or PDFs scanned into the patient’s electronic health record (EHR) for relevant and necessary patient information.
We need discrete, transferable data that can be imported into EHRs. This high-stakes data must be more shareable, interoperable, and accessible from any future treatment site.
To treat patients effectively, clinicians must be able to prescribe the appropriate therapies and provide necessary care rapidly and without redundancy.
We need discrete, transferable data that can be imported into EHRs. This high-stakes data must be more shareable, interoperable, and accessible from any future treatment site.
2.Research recruitment
Researchers continue to investigate numerous innovative approaches and tools to improve HIV prevention and treatment.
This presents its own set of challenges, one of which is the recruitment of clinical study participants.
Research studies are often based in large medical centers, and eligible individuals who receive care elsewhere may be unaware of, or may not have access to, ongoing trials.
Such challenges may be associated with research recruitment delays or narrower participant pools, slowing the development of safe and effective interventions.
Researchers continue to investigate numerous innovative approaches and tools to improve HIV prevention and treatment.
This presents its own set of challenges, one of which is the recruitment of clinical study participants.
Such challenges may be associated with research recruitment delays or narrower participant pools, slowing the development of safe and effective interventions.
Oracle has partnered with the Fred Hutchinson Cancer Center to develop the HIV Red Ribbon Registry (RRR) to facilitate participant recruitment for HIV vaccine trials.
The RRR is a database of people interested in supporting HIV clinical research by participating in studies.
This digital recruitment solution enables more efficient recruitment of participants for HIV vaccine trials, an often prolonged and laborious process.
Streamlining researchers’ ability to reach a wider audience for HIV trial recruitment translates to more representative data in study cohorts and, ultimately, accelerated innovation.
Streamlining researchers’ ability to reach a wider audience for HIV trial recruitment translates to more representative data in study cohorts and, ultimately, accelerated innovation.
3.Cross-clinic collaboration
We spoke with Alfredo Guzman, director of technology and data services for the University of Alabama at Birmingham (UAB) 1917 Clinic, about the Data for Care (D4C) initiative.
D4C leverages data from seven Ryan White clinics in Alabama to enhance retention in HIV care, a key component of the care continuum.4
D4C uses an evidence-based intervention in which patients are classified as low-, medium-, or high-risk depending on the number of missed clinic visits in the prior year.
Medium- and high-risk patients receive personal reminders before their clinic visits and within 48 hours of a missed visit.
D4C uses an evidence-based intervention in which patients are classified as low-, medium-, or high-risk depending on the number of missed clinic visits in the prior year.
Medium- and high-risk patients receive personal reminders before their clinic visits and within 48 hours of a missed visit.
With busy schedules and other demands often interfering with personal health, D4C aims to keep patients engaged and retained in care so they can achieve viral suppression.
Though this initiative may sound simple, providing this high-touch, personalized patient support requires intentional effort and is only possible with accessible, complete, and actionable patient data.
4.Public health systems
Patient drop-off at each step of the HIV care continuum implicates a different set of challenges for public health and care delivery.
To fully understand the barriers to care that PLHIV experience, we must effectively capture and analyze comprehensive data about this population and the environments in which they seek care.
Data is collected at individual clinics and within organizations across the country, but to understand trends across a population, they must be centrally reported in a standardized format that facilitates aggregation and analysis.
Testing and care data must account for each patient’s medical history (e.g., new vs. old diagnosis) and include relevant demographics that enable local districts to understand the epidemiology of transmission in the population.
Furthermore, local districts and organizations must have direct access to population-level data, so they aren’t reliant on state- and national-level analyses to quickly respond to the needs of their communities.
To fully understand the barriers to care that PLHIV experience, we must effectively capture and analyze comprehensive data about this population and the environments in which they seek care.
Many clinics and health departments across the country still rely on paper records for these processes.
Others utilize siloed or disjointed systems.
We’re only able to effectively direct resources such as testing events, transportation support, and medication assistance programs if we know where the problems lie.
Additionally, at a time when our healthcare and public sector workforce is facing unprecedented burnout, we need digital tools that make these processes easy.
Many clinics and health departments across the country still rely on paper records for these processes. Others utilize siloed or disjointed systems.
Additionally, at a time when our healthcare and public sector workforce is facing unprecedented burnout, we need digital tools that make these processes easy.

Thinking about data on a continuum
The HIV care continuum enables us to view the persisting gaps in our care system.
We utilize a continuum framework to better understand the unique challenges and pitfalls associated with each step in the care experience.
We can also consider how a similar framework could help us understand the specific challenges associated with each stage of data capture, sharing, analysis, and application — a data continuum.
Necessary cross-sector enhancements include increased data standardization across different data capture systems and better, less siloed tools to decrease duplication of effort and human error.
- We need digital systems that facilitate data sharing and cross-facility communication while adhering to best practices for safeguarding personal health information.
- We need systems that support vertical data analysis to facilitate utilization at the local, state, national, and global levels.
- We need systems co-developed alongside the health professionals who utilize them to improve functionality, increase efficiency, and decrease burnout across the workforce.
- We need data systems that reveal where our healthcare falls short to inform policy and interventions that will maximize health systems’ return on investment and improve health.
We must work to enhance each step of this data continuum and provide healthcare and public health professionals with optimized tools that will help us succeed in the fight against HIV/AIDS.
The job isn’t done; we have much to do if we want to truly eradicate one of the world’s most serious public health challenges.
Necessary cross-sector enhancements include increased data standardization across different data capture systems and better, less siloed tools to decrease duplication of effort and human error.
We must work to enhance each step of this data continuum and provide healthcare and public health professionals with optimized tools that will help us succeed in the fight against HIV/AIDS.
The job isn’t done; we have much to do if we want to truly eradicate one of the world’s most serious public health challenges.
References
- Eisinger RW, Dieffenbach CW, Fauci AS. HIV Viral Load and Transmissibility of HIV Infection: Undetectable Equals Untransmittable. JAMA. 2019 Feb 5;321(5):451–452. doi: 10.1001/jama.2018.21167. PMID: 30629090.
- CDC, Monitoring selected national HIV prevention and care objectives by using HIV surveillance data — United States and 6 dependent areas, 2019. HIV Surveillance Supplemental Report 2021;26(№2). Published May 2021. https://www.hiv.gov/federal-response/policies-issues/hiv-aids-care-continuum. Last accessed November 22, 2022.
- Schafer KR, et al. The Continuum of HIV Care in Rural Communities in the United States and Canada: What Is Known and Future Research Directions. J Acquir Immune Defic Syndr. 2017 May 1;75(1):35–44. doi: 10.1097/QAI.0000000000001329. PMID: 28225437; PMCID: PMC6169533.
- Sohail M, et al. Data for Care (D4C) Alabama: Clinic-Wide Risk Stratification With Enhanced Personal Contacts for Retention in HIV Care via the Alabama Quality Management Group. J Acquir Immune Defic Syndr. 2019 Dec;82 Suppl 3:S192-S198. doi: 10.1097/QAI.0000000000002205. PMID: 31764254.
About the authors & affiliations
Virginia Burke
Virginia currently works as a Health Innovation Scientist within the Innovation Team at Oracle Health.
She has worked in a variety of international and domestic public health settings over the past ten years, previously leading a variety of USAID-, NIH-, and WHO-funded HIV/AIDS research studies in sub-Saharan Africa and the U.S. At the start of the COVID-19 pandemic, she was responsible for strategically developing, scaling, and managing emergency pandemic response teams at the local health district level in Richmond, Virginia. She brings expertise in infectious disease response, mixed methods research and evaluation, telehealth, and managing local and global partnerships with academic, NGO, and governmental stakeholders.
Virginia received her B.A. from the University of Virginia in Global Development Studies and her Master of Science in Public Health from Johns Hopkins Bloomberg School of Public Health, specializing in international health social and behavioral interventions. She currently lives in Richmond, VA with her husband, daughter, and yellow lab.
James Willig, MD, MSPH
James H. Willig, MD, MSPH, is the Associate Dean of Clinical Education at the University of Alabama at Birmingham School of Medicine.
He attended Medical School at the Instituto Tecnologico de Santo Domingo (INTEC) and completed his residency at the University of Virginia Roanoke-Salem. At UAB, Willig has earned an M.S. in Public Health and completed an Infectious Diseases Fellowship. His research interests in quality improvement and medical informatics have found expression in both research into quality issues in HIV primary care and the design of software to enhance research and education.
Dr. Willig is a visiting scientist with the Oracle Health and Innovation team from September 2022-March 2023. During his six-month tenure, Dr. Willig works alongside the Health and Innovation Team on multiple ongoing projects focused on new approaches to diagnostics and other aspects of healthcare. While in this temporary role, Dr. Willig hopes to learn more about Oracle’s approach to healthcare, and to work with the interdisciplinary talent on the Health and Innovation team and throughout Oracle to explore collaborative opportunities to optimize healthcare delivery. “None of us is as smart as all of us” is one of his favorite phrases and finding new opportunities in the interstitial space between disciplines is something he truly enjoys.
Originally published at https://blogs.oracle.com.












