The Lancet Commentary
Carolina Batista
Peter Hotez
Yanis Ben Amor
Jerome H Kim
David Kaslow
Bhavna Lall
Onder Ergonul
J Peter Figueroa
Mayda Gursel
Mazen Hassanain
Gagandeep Kang
Heidi Larson
Denise Naniche
Timothy Sheahan
Annelies Wilder-Smith
Shmuel Shoham
Samba O Sow
Prashant Yadav
Nathalie Strub-Wourgaft
Sarah-Jane Loveday
Emma Hannay
Maria Elena Bottazzi
December 13, 2021
who
The COVID-19 pandemic is a public health crisis of unprecedented proportions.
Despite unparalleled rates of vaccination and mass testing in certain parts of the world, the virus rages on.
Since SARS-CoV-2, the virus that causes COVID-19, was first isolated in Wuhan, China, on 7 January 2020, more than 243 million cases and 4·9 million deaths have been reported worldwide at the time of writing.
Widespread testing and timely diagnosis are critical for pandemic control and preparedness.
This is especially true for SARS-CoV-2, as asymptomatic and presymptomatic individuals play a key role in spreading the virus.
Understanding COVID-19 epidemiology in each region of the world is essential to guide policy; however, this is not always feasible …
… due to limited access to tests, poor laboratory infrastructure, insufficient personnel and strained health systems.
In addition, the so-called COVID-19 “infodemic” has contributed to the dissemination of anti-science messages and negatively influenced testing and vaccine uptake worldwide.
Alarming inequities have set the tone of this pandemic.
Low- and middle-income countries (LMICs) have struggled to access existing tools to ease the impact of COVID-19 on their fragile health systems.
By September 2021, 5·82 billion vaccine doses had been administered, although just 1·9% of individuals in LMICs had received at least one dose, while of the more than 3·2 billion tests performed worldwide, just 0·4% were in LMICs.
Of the various types of tests available for SARS-CoV-2, antigen rapid diagnostic tests (Ag-RDTs) represent the most promising tools for the scale-up of testing.
Ag-RDTs combine good performance with a rapid turnaround time, while not requiring sophisticated laboratory infrastructure or skilled personnel.
This facilitates decentralized testing, so Ag-RDTs could potentially accelerate detection and responses to COVID-19 at scale in LMICs to help control the pandemic.
When the first Ag-RDTs received World Health Organization (WHO) emergency use listing (EUL) in September 2020, global institutions called for improved access and affordability of these tests for LMICs.
Since then, multiple tests have been developed and brought to market.
Independent evaluation and real-time results sharing are essential to ensure quality standards are met and to build diagnostics literacy to guide countries’ testing policies.
FIND, the global alliance for diagnostics and others have played a key role in conducting such studies.
The role of diagnostics has evolved throughout the COVID-19 crisis and will continue to do so.
Enhanced access to testing will improve surveillance, generate real-world vaccine efficacy data, guide strategies, and enable emerging variants to be monitored.
As clinical trials targeting outpatients progress, improved access to tests will support demand-forecasting efforts and enable treatment adoption.
The pandemic has exposed the inadequacy of global supply chains to ensure access to the tools countries need to fight COVID-19.
The reliance on a few diagnostics manufacturers has generated unfair competition for scarce resources and jeopardized the capacity of LMICs to scale-up testing coverage.
While medicines and vaccines require complex technology transfer mechanisms, simple lateral flow tests can be easily manufactured in regions where test production is currently limited.
Regional diversification of manufacturing capacity is urgently needed to contribute to countries’ resilience and preparedness.
Ongoing technology transfer agreements will expand regional capacity through partnerships and help ensure equitable access to diagnostics in LMICs.
The emergence of variants of concern has highlighted the importance of genomic epidemiology and sequencing.
However, the sequencing of new variants requires training, infrastructure and sustained reagent supply; both short- and long-term commitments are needed to reinforce regional capabilities to achieve this (see Panel 1).
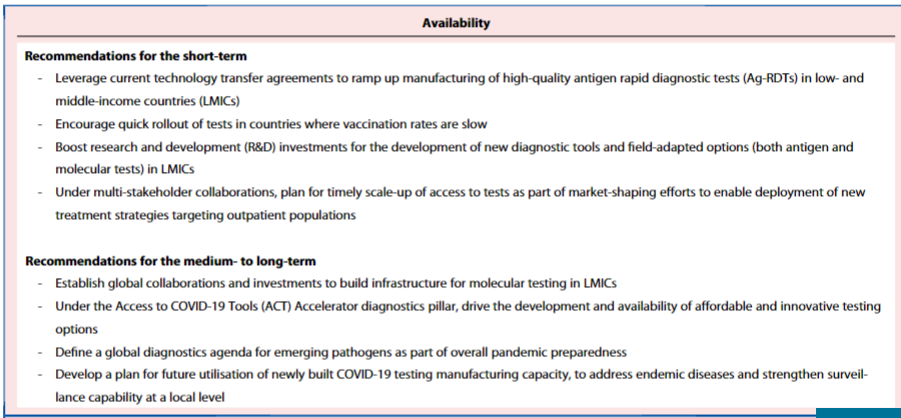
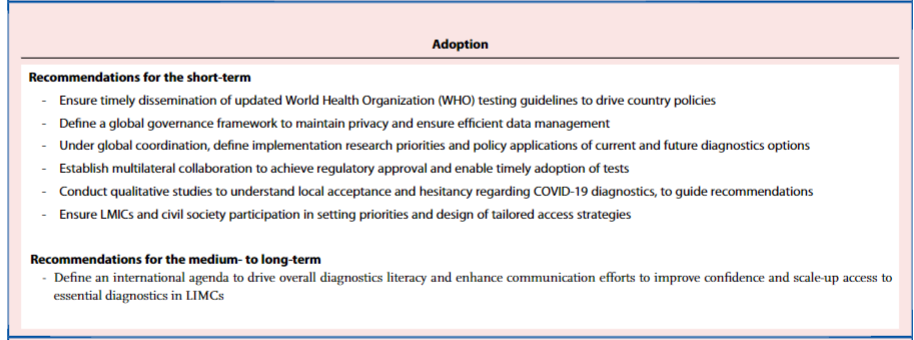
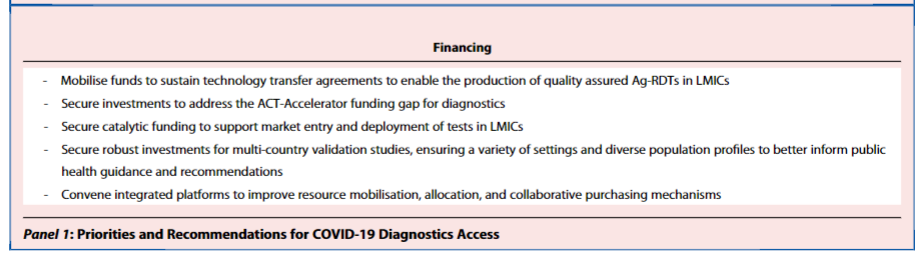
There is an urgent need for further investments to develop and scale-up access to diagnostics… ,
… as LMIC populations have been uniquely vulnerable yet invisible, with their undocumented infections unable to demand the requisite attention or priority.
The COVID-19 pandemic has underscored the chronically poor testing capacity in resource-limited settings and the need for improved national diagnostics strategies.
It is critical that testing capabilities are increased to identify surges, assess vaccine effectiveness, promote innovative approaches including test and treat strategies, and to identify and monitor emerging variants.
The speed of scientific progress to develop tools to tackle COVID-19 has been remarkable.
Such progress would not have been possible without collaboration, robust public and private investments and effective coordination.
However, these achievements have not been made available equitably, and huge access asymmetries remain between rich and poor countries. ]
Testing remains an essential pillar to help fight the COVID-19 pandemic and to ensure preparedness for future pandemics.
Authors & affiliations

a Médecins Sans Frontières, Rio de Janeiro, Brazil
b Texas Children’s Center for Vaccine Development, Baylor College of Medicine, Houston, TX, USA
c Center for Sustainable Development, Columbia University, New York, New York, United States of America
d International Vaccine Institute, Seoul, South Korea
e Program for Appropriate Technology in Health (PATH) Essential Medicines, PATH Seattle, WA, USA
f University of Houston College of Medicine, Houston, TX, USA
g Koc University Research Center for Infectious Diseases, Istanbul, Turkey
h University of the West Indies, Mona, Kingston, Jamaica
i Middle East Technical University, Ankara, Turkey
j College of Medicine, King Saud University, Riyadh, Saudi Arabia
k Christian Medical College, Vellore, India
l London School of Hygiene & Tropical Medicine, London, UK
m ISGlobal-Barcelona Institute for Global Health-Hospital Clinic-University of Barcelona, Spain
n University of North Carolina, Gillings School of Global Public Health, Chapel Hill, NC, USA
o Heidelberg Institute of Global Health, University of Heidelberg, Heidelberg, Germany
p Johns Hopkins University School of Medicine, Baltimore, MD, USA
q Center for Vaccine Development, Bamako, Mali
r University of Maryland, MD, USA
s Center for Global Development, Washington, DC, USA
t Harvard Medical School, Boston, MA, USA
u Technology and Operations Management, INSEAD
v Drugs for Neglected Diseases Initiative, Geneva, Switzerland
w FIND, the global alliance for diagnostics, Geneva, Switzerland
CB, S-JL, EH wrote the initial draft. MEB and BL managed the process of review. All authors contributed equally and provided critical feedback, reference sources, and critical revisions for intellectual content and verified the information presented here.
Lancet Commission on COVID-19 Vaccines and Therapeutics Task Force
References
See the original publication
Declaration of Interest
See the original publication
Acknowledgments
We thank Jeffrey Sachs, Chair of the Lancet COVID-19 Commission for his invaluable review and feedback.
Originally published at https://www.thelancet.com.